The first domestically produced biosimilar drug was given to the five major monoclonal antibodies.
According to the latest news from the medical website on January 31, the application for the listing of Shanghai Fuhong Hanlin Rituximab Injection has been reviewed and is currently in the approval stage. If there is no accident, it will be approved soon. It will also be the first domestic biosimilar drug to be in line with international standards.
In recent years, the development of biosimilar drugs in the world has focused on a series of biological agents that have expired or are about to expire, including adalimumab, infliximab, rituximab, bevacizumab, trastuzum Monoclonal antibody, etc. The monoclonal antibody drugs have developed rapidly in the global market, and domestic pharmaceutical companies have also been deployed. This competition is competing for this fast-growing field, and Chinese biosimilar drugs have ushered in a good era of vigorous development.
The development trend of domestic biosimilar drugs is very good. The following focuses on several domestic heavy monoclonal antibody varieties that are in the stage of application for listing.
status quo
Good policy
In recent years, China has formulated and promulgated a number of biosimilar drug-related policies, hoping to use biosimilar drugs to break the status quo of long-term dependence on imports of monoclonal antibodies. In December 2014, the State Food and Drug Administration promulgated the “Guidelines for the Development and Technical Guidance of Biosimilar Drugs (Draft for Comment)â€; in February 2015, the “Guidelines for the Development and Technology of Biosimilar Drugs (Trial)†was promulgated; In July, the “Regulations on the Administration of Drug Registration (Revised Draft)†further standardized the concept and approval criteria for biosimilar drugs; in October 2017, the “Opinions on Deepening the Reform of Examination and Approval System and Encouraging Innovation in Drug Medical Devices †jointly issued by the two offices Once again, it is explicitly proposed to support the development of biosimilar drugs.
The successive introduction of these policies has extremely important guiding significance for the development of biosimilar drugs in China, and it also indicates that China's biosimilar drug approval policies have gradually been in line with international standards.
Hundred flowers
At present, there are many biopharmaceuticals in China that are in the stage of submitting for listing. The main products are rituximab-like drug HLX01 from Qihong Hanlin and bevacizumab-like drugs Qilu Pharmacology QL1101 and Sansheng Guojian. The trastuzumab is similar to the recombinant anti-HER2 humanized monoclonal antibody (302H), and the infliximab-like drug CMAB-008 of Baimaibo. In addition, adalimumab-like drugs are at the stage of listing application, including BAT1406 of Baiaotai, HS016 of Hisun Pharmaceutical, IBI303 of Cinda Bio, and HLX03 of Fuhong Hanlin, and products of many companies are already in III. Clinical phase.

Key variety
Rituximab
Original research company: Roche
Domestic submission of listing application: Fu Hong Hanlin
Rituximab was developed by Roche under the trade name "Rituxan" and was approved by the FDA and EMA in 1997 and 1998. The main indications are non-Hodgkin's lymphoma, chronic lymphocytic leukemia and rheumatoid arthritis. Currently, the European Union has approved two similar drugs for rituximab: Truxima from Celltrion and Rixathon/Riximyo from Sandoz, Novartis.
Global Market
According to the statistics of global best-selling drugs, the sales of rituximab in 1998 was 320 million US dollars, the sales in 2007 exceeded 5 billion US dollars, the sales in 2017 reached 7.5 billion US dollars, and the sales in 2007-2017 have exceeded 50 for 11 consecutive years. One hundred million U.S. dollars.
From the sales situation in 1998-2017, the cumulative sales of this product is as high as 95.8 billion US dollars, and it is still the dominant product in the field of CD20 monoclonal antibody. In the first three quarters of 2018, rituximab had global sales of $5.093 billion and expected annual sales of $6.8 billion.
Domestic market
In 2000, Roche's rituximab was imported and marketed in China. The product name was rituximab, and the dosage form was injection. The specifications were 100mg/10ml, 500mg/50ml, and the indications for non-Hodgkin's lymphoma were approved in China.
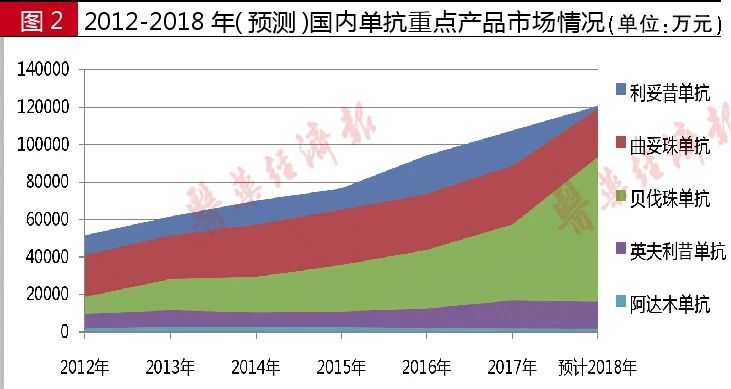
According to the statistics of domestic sample hospitals , the amount of rituximab used in 2012 was 516 million yuan, and in 2017 it was 1.08 billion yuan. In the first three quarters of 2018, the amount of drug used in domestic sample hospitals was 910 million yuan. It is expected that the amount of drugs used in 2018 will exceed 1.20 billion yuan. This product is Roche's best-selling drug in China. After the 2017 medical insurance access negotiations, the price reduction of rituximab was as high as 58.45%, and the market quickly expanded.
Declaration status
At present, there are more than a dozen domestic rituximab-like drug reporting companies, and the fastest research and development progress is Fuhong Hanlin, which is already in the application stage. In addition, the products of Cinda Bio, Shenzhou Cell Engineering, Xikang Bio, and Hisun Pharmaceutical entered the clinical phase III, and CITIC Guojian withdrew.
In January 2018, the application for the treatment of non-Hodgkin's lymphoma and rheumatoid arthritis by Fuhong Hanlin rituximab injection was included in the priority review.
Review
This is the first biosimilar drug to be applied for in China. It was also expected to be the first domestically produced biosimilar drug to enter the market. It is currently approved.
Trastuzumab
Original research company: Roche
Domestic submission of listing application: Sansheng Guojian
Trastuzumab was produced by Roche, under the trade name "Hercepti", approved by the FDA in 1998 and subsequently approved in Europe and Japan in 2000 and 2001 respectively.
Currently, there are only two types of trastuzumab-like biosimilar drugs listed worldwide: FDA approved Ogivri developed by Mylan/Biocon and Ontruzant approved by Samsung EMA.
Global Market
According to statistics of global best-selling drugs, the sales of trastuzumab in 1999 was 188 million US dollars, the sales in 2003 exceeded 1 billion US dollars, the sales in 2006 exceeded 3 billion US dollars, the sales in 2010 exceeded 5 billion US dollars, 2017 Sales were $7.127 billion. In 1997-2017, the cumulative sales of this product reached $70.5 billion. In the first three quarters of 2018, trastuzumab global sales were $5.289 billion, with annual sales expected to be $7.1 billion.
Domestic market
In September 2002, Roche's trastuzumab was approved for entry into China under the trade name Herceptin. It was approved for the treatment of HER2-positive breast cancer and HER2-positive gastric cancer in China. The dosage form was powder injection, and the specifications were 440mg and 150mg.
According to the statistics of domestic sample hospitals, the amount of trastuzumab in 2012 was 410 million yuan. In 2016, the amount of medicine used was 737 million yuan. In 2017, the amount of medicine used was 887 million yuan, an increase of 20.4% over the same period. In the first three quarters of 2018, the overall market for trastuzumab was 890 million yuan. It is estimated that the amount of medicine used in 2018 will exceed 1.20 billion yuan. In 2017, trastuzumab entered the medical insurance catalog through price negotiations, and the price reduction reached 65%. In 2018, the number of base drugs was expanded, and trastuzumab entered it.
Declaration status
At present, there are more than 10 companies that have applied for trastuzumab-like drugs in China, and Sansheng Guojian, which has the fastest research and development progress, has submitted a listing application, and the trade name is Saiping. In addition, the products of Jiahe Biological, Zhengda Tianqing, Fuhong Hanlin, Anke Bio and Haizheng Pharmaceutical entered Phase III clinical trials.
Review
Sansheng Guojian's trastuzumab biosimilar drugs are expected to be approved for the first time. With the continuous expansion of medical insurance coverage, the market size is expected to grow rapidly in the future.
Adalimumab
Original research company: Aberdeen
Domestic application for listing: Biotech, Hisun Pharmaceutical, Cinda Bio, Fuhong Hanlin
Adalimumab was developed by Abbott, under the trade name "Humira", approved by the FDA in December 2002, approved by the EMA in September 2003, and approved by PMDA in April 2008. Approved indications mainly include ankylosing spondylitis, Crohn's disease, psoriasis, juvenile idiopathic arthritis, psoriatic arthritis, ulcerative colitis, skin suppurative sweat gland inflammation, uveitis. The drug has been approved for marketing in more than 90 countries around the world.
Currently, the United States has approved three similar drugs for adalimumab: Amjevita from Amgen, Cyltezo from Boehringer Ingelheim, and Hyrimoz from Sandoz. The EU has approved four: Amgevita and Cyltezo, as well as Amgen's Solymbic and Samsung's Imraldi.
Global Market
According to statistics of global best-selling drugs, sales of adalimumab in 2003 was 280 million US dollars, over 1 billion US dollars in 2005, over 2 billion US dollars in 2006, over 5 billion US dollars in 2009, over 10 billion US dollars in 2013, 2016 Over $15 billion, 2017 sales of $18.908 billion. In 2003-2017, the cumulative sales of this product has reached US$115.9 billion, and it has been sitting on the global “King of Medicine†for six consecutive years. In the first three quarters of 2018, adalimumab had global sales of $15.018 billion and is expected to have annual sales of more than $20 billion.
Domestic market
In 2009, Aberdeen's adalimumab was approved for marketing in China. The product name is Xiumele, and the dosage form is injection. The specification is 140mg/0.8ml.
According to statistics from domestic sample hospitals, the amount of drug used in adalimumab in 2012 was 18.09 million yuan, compared with 22.08 million yuan in 2016 and 17.95 million yuan in 2017, down 11.5% from the same period. In the first three quarters of 2018, the amount of adalimumab was 12.09 million yuan. It is estimated that the amount of medicine used in 2018 will exceed 17 million yuan.
Compared with the global market, the current sales of adalimumab in China is not ideal. As more indications are listed in China, the market potential is still huge.
Declaration status
At present, there are more than 20 domestic companies that declare adalimumab-like drugs. The fastest research and development progress is BIOTEC, Haizheng, Cinda Biology and Fuhong Hanlin, which are already in the application stage. In addition, Junshi Biological and Zhengda Tianqing's products entered the clinical phase III.
Review
The battle for the four domestic enterprises to compete for the first imitation qualification of adalimumab biosimilar drugs has become increasingly fierce. Judging from the progress of the declaration, Baiaotai is faster than Haizheng, Haizheng is faster than Xinda, and Fuhong Hanlin has only declared production in recent days. Once the first adamu biosimilar drug goes on the market, it will have a place with a price advantage.
Bevacizumab
Original research company: Roche
Domestic submission of listing application: Qilu Pharmaceutical, Cinda Bio
Bevacizumab was developed by Roche's Genentech under the trade name "Avastin". The drug was approved by the FDA and EMA in February 2004 and January 2005. At present, a number of solid tumor indications such as colorectal cancer, non-small cell lung cancer, glioblastoma, renal cell carcinoma, cervical cancer, ovarian cancer, fallopian tube cancer, and peritoneal cancer have been obtained.
Currently, there is only one type of bevacizumab-like biosimilar drug in the global market, namely Mvasi jointly developed by Anjin and Aijian, which has been approved in the US and EU.
Global Market
According to statistics of global best-selling drugs, the sales of bevacizumab in 2005 was 188 million US dollars. In 2002, the sales exceeded 1 billion US dollars. In 2006, it exceeded 3 billion US dollars. In 2010, it exceeded 5 billion US dollars. After 8 consecutive years. Sales range from $6 billion to $7 billion.
The drug's cumulative sales in 1999-2017 was $71.2 billion. In the first three quarters of 2018, bevacizumab sales in the global market were $5.009 billion, with annual sales expected to be $6.7 billion.
The European patent protection of bevacizumab expires in 2018, and the US patent protection will expire in 2019. The drug has become a popular imitation product at home and abroad.
Domestic market
In February 2010, Roche's bevacizumab was approved for marketing in China under the trade name Avastin, which is mainly used for the treatment of metastatic colorectal cancer and advanced, metastatic or recurrent non-small cell lung cancer. The dosage form is an injection solution, and the specifications are 100 mg (4 ml) and 400 mg (16 ml).
According to the statistics of domestic sample hospitals, the amount of bevacizumab in 2012 was 186 million yuan, 282 million yuan in 2013, 434 million yuan in 2016, and 573 million yuan in 2017, an increase of 30.9% over the same period. In the first three quarters of 2018, the amount of bevacizumab was 700 million yuan, and it is expected to exceed 950 million yuan in 2018.
In 2017, Bevalizumab entered the national medical insurance catalogue through price negotiations, and the price reduction rate reached 62%. With the release of the medical insurance policy dividend, the market grew rapidly.
Declaration status
At present, there are more than 20 companies that have reported bevacizumab-like drugs in China. The fastest progress in research and development is Qilu Pharmaceutical, which is already in the application stage. On January 29, Cinda Biotech also announced that its bevacizumab biosimilar IBI-305 application has been accepted. In addition, the products of Dongpu Pharmaceutical, Beijing Tianguang Shibi, Zhengda Tianqing, Hengrui, Luye Pharmaceutical, Baiaotai and Jiahe Biological entered Phase III.
Review
Qilu Pharmaceutical's recombinant anti-VEGF humanized monoclonal antibody injection was included in the 33rd batch of priority review list as a major special product. If you can get the top spot, it will help promote the rapid growth of the company's cancer product line.
Infliximab
Original research enterprise: Johnson & Johnson
Domestic submission of listing application: Baimaibo
Infliximab was developed by Jansson, a Johnson & Johnson company, under the trade name "Remicade", and was approved by the FDA and EMA in 1998 and August 1999. Currently approved for the treatment of inflammation-related diseases, including Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and plaque psoriasis. The drug's European patent expired in 2014 and the US patent expires in 2018.
Currently, there are only three infliximab biosimilars approved in the United States: Pfizer/Celltrion's Inflectra, Pfizer's Ixifi, and Samsung's Renflexis. EMA approved three products, Pfizer's Inflectra, Celltrion's Remsima and Samsung's Flixabi.
Global Market
According to statistics of global best-selling drugs, the sales of infliximab in 1999 was US$146 million. In 2002, it exceeded US$1.5 billion. In 2003, it exceeded US$2 billion. In 2005, it exceeded US$3 billion. In 2007, it exceeded US$5 billion. Sales for the 10 consecutive years ranged from $6 billion to $8 billion, with sales in 2017 of $8.216 billion.
Domestic market
In 2007, Johnson & Johnson's infliximab was imported and marketed in China. The product name was gram, the dosage form was powder injection, and the specification was 100mg. The indication for psoriasis was approved in China.
According to statistics from domestic sample hospitals, Infliximab had sales of 95.61 million yuan in 2012, sales of 124 million yuan in 2016, and sales of 168 million yuan in 2017, an increase of 35.3% over the same period. In the first three quarters of 2018, the amount of medication used in the domestic sample hospitals of Infliximab was 120 million yuan. It is estimated that the amount of medicine used in 2018 will exceed 160 million yuan.
Declaration status
At present, among the enterprises that declare infliximab biosimilar drugs in China, the fastest progress in research and development is Baimaibo, which is already in the stage of application for listing. In addition, the products of Haizheng Pharmaceutical and Jiahe Biological entered the clinical phase III.
Review
The product has not entered the national health insurance catalogue and is expensive, and it has not been approved for the treatment of rheumatoid arthritis alone, and its clinical use is limited.
Torch Shaped Wafer Ice Cream Cone
Torch Shaped Wafer Ice Cream Cone,Wafer Products In The Shape Of Torch,Wafer Cone Filled With Soft Ice,Flat Mouth Conical Wafer Products
Tianjin Yongkang Food Co., Ltd , https://www.yongkangfood.com
