Twirling for twenty years! The next breakthrough in RNAi drug development is here
Twirling for twenty years! The next breakthrough in RNAi drug development is here
March 12, 2019 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)]; The RNA interference (RNAi) pathway regulates mRNA stability and translation in almost all human cells. Small double-stranded RNA molecules can effectively trigger RNAi silencing of specific genes, but their therapeutic applications face many challenges related to safety and efficacy. However, August 2018 marked a new era in the field, and the US Food and Drug Administration (FDA) approved the first RNAi-based drug Onpattro (patisiran).
Recently, three researchers from the Department of Molecular and Cell Biology of the Beckman Institute in Hope, USA, Ryan L. Setten, John J. Rossi, and Si-ping Han published a review article on Nature to discuss RNAi drug design. Key developments in development and development, current state of clinical pipelines, and prospects for future advances, including novel RNAi pathway preparations using mechanisms other than post-translational RNAi silencing.
The article states that in order to utilize the mammalian RNAi pathway to efficiently and specifically inhibit putative therapeutic targets, RNAi pharmaceutical preparations must overcome pharmacodynamic-related challenges (target specificity, off-target RNAi activity, immunosensor-mediated cytotoxicity) and Pharmacokinetic related challenges (circulatory system, cellular uptake, endosomal escape). These challenges are currently being addressed by the structural motifs of RNAi triggers, sequence selection, chemical modeling, and the selection and engineering of delivery routes and excipients.
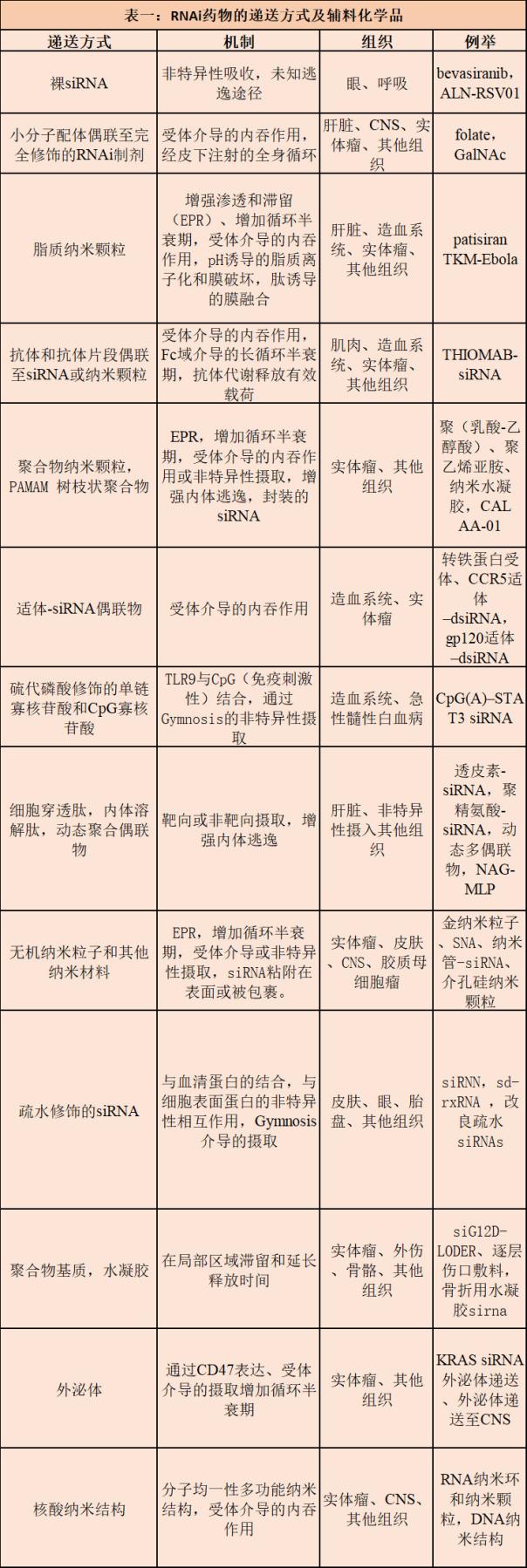
The approval of the current clinical RNAi pipeline patisiran heralds a dynamic new era of RNAi therapy, in which safe and effective RNAi payload and delivery strategies are passing more mature clinical research and development and current Good Manufacturing Practices (cGMP) The pipeline, which is producing promising drugs for a variety of indications for liver and non-liver tissue.
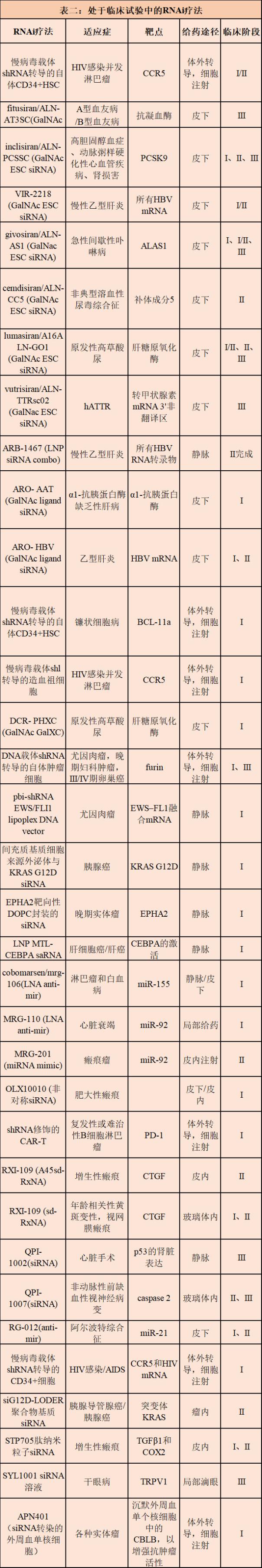
Currently, several drug candidates for liver and non-liver indications are in Phase I, Phase II, and Phase III clinical trials, and new research drugs (IND) for the central nervous system and other non-liver tissues are about to enter the clinic. . In addition, new technologies for RNAi payloads and delivery excipients are likely to develop in more tissues and indications over the next five years. All in all, these advances indicate that RNAi treatment will have many ways to make fruitful innovations over the next decade.
Experiences and Lessons from RNAi Drug Development The advances and setbacks in the preclinical and clinical development of RNAi drugs have made the RNAi drug development process increasingly mature and savvy. The main lessons include: the benefits of empirical testing, the continued evolution of Dianophores (determining the molecular characteristics of pharmacokinetics) and Pharmacophores (determining the molecular characteristics of pharmacodynamics), and the importance of predicting safety and drug activity using the correct animal models. Sexuality, the need to minimize excipient complexity and toxicity to simplify production, storage and clinical testing and reduce the risk of major adverse events.
Although the number of common base and backbone chemical molecules of RNAi flip-flops is unbelievably low, the presence of more than 20 modifiable nucleotides in each strand of an RNAi flip-flop results in a possible modification of the astronomical number. Through empirical screening, the generational development of Alnylam's ESC technology and its corresponding increase in clinical potency and safety have shown the power and potential of evolutionary development. With the emergence of more RNAi-compatible bases and backbone chemical molecules, the development of Dianophores will continue to be strong.
It is important to accurately predict toxicity and efficacy using the correct in vitro and in vivo models when performing empirical testing. Different model organisms respond differently to oligonucleotides and excipients. Other important considerations are the complexity, uniformity, stability, and toxicity of excipients such as nanoparticles, polymers, peptides, and proteins. Lipids and polymer nanoparticles have been widely used to improve pharmacokinetic properties, but their preparation is challenging, and their products tend to have a degree of heterogeneity in particle composition, particle properties and drug loading, making It is difficult to establish a treatment window during clinical development.
In addition, after storage or administration, the particles may become unstable and release decomposition products, resulting in an untrackable toxicity. Similarly, although endoplasmic membrane-dissolving excipients (such as melittin) can significantly improve endosomal escape of RNAi agents, they can also be very toxic.
Ongoing challenges for RNAi drugs to respond <br> Despite advances in clinical RNAi drug development, there is still much room for improvement in pharmacokinetics, pharmacodynamics, and limiting toxicity strategies. In order to achieve these goals, continuous progress has been made in mature technologies such as polymer nanoparticles (LNP), aptamers, molecular ligands and oligonucleotide bases and backbone modifications.
In addition, there are new approaches that are expected to overcome existing challenges in systemic and local RNAi administration, specificity and safety of RNAi payloads, including the use of reverse transport strategies to improve endosomal escape, antibody and siRNA Improve pharmacokinetics and achieve tissue-specific targeting, enhance siRNA potency through hydrophobic modification, reduce lipid nanoparticle toxicity using biodegradable ionized lipids, perform whole body siRNA delivery using exosomes, and utilize layer-layer Electrostatic components and injectable biodegradable hydrogels enhance local delivery, improve thermodynamic stability of nucleic acid nanostructures by cross-linking modified nucleic acids, rapidly reverse RNAi activity by developing antagonists, conditional RNAi activity using dynamic nucleic acid nanotechnology, using stem cells And three-dimensional culture to prepare organoids to replace preclinical models.
In addition to siRNA, an in-depth study of miRNA-induced RNAi mechanisms has resulted in synthetic miRNA therapies and constructs, collectively referred to as anti-mirs and blocking mirs, which inhibit the activity of specific miRNAs or block the silencing of specific miRNA targets, respectively. In addition, other functions of short dsRNA have been discovered.
For example, a guide RNA produced by a small dsRNA can induce gene silencing or gene activation at an transcript level in an Ago-dependent manner. The maturity of the following technologies based on these findings is expected to greatly increase the number of disease indications that can be treated by future RNAi therapies, including: miRNA mimics, miRNA inhibitors (anti-mirs), miRNA competition (blocking-mirs) agonists, Small dsRNA-mediated transcriptional gene silencing, small dsRNA-mediated transcriptional gene activation.
Conclusion The approval of Onpattro has made the systematic delivery of RNAi therapy to the liver a clinical reality. Although GalNAc-conjugated, metabolically stable siRNA follow-up drugs are still in clinical development, the continued efficacy and safety of these drugs in different liver indications is quite optimistic, developing for indications for the eye, kidney and central nervous system. The same is true for other RNAi therapies. Many clinical drug candidates currently target rare diseases, but if approved, Alnylam's PCSK9 inhibitors inclisiran and Quark's QP-1002 for kidney damage and PF-655 for wet AMD may affect a larger patient population.
At present, RNAi therapy has been successful, and it is clear that if systemic delivery to non-liver, non-kidney tissue becomes feasible in clinical applications, the impact of RNAi therapy may be greatly expanded.
Key challenges include avoiding clearance of kidney and reticuloendothelial tissue, enhancing uptake in cell types that do not have high expression load internalization receptors, and improving endosomal escape. Some of these issues may apply to methods that work more closely with complex biological pathways, such as those responsible for intracellular sorting and endocytic transport. This requires a closer collaboration between chemists and biologists than in the past to develop solutions that exploit the subtle aspects of biological pathways.
In addition, it may be desirable to use chemically complex types of multifunctional excipients that have so far plagued the clinical development of RNAi-carrying nanoparticles. Alternatively, metabolically stable RNAi triggers and conjugated ligands are further developed to efficiently deliver to more and more extrahepatic tissues without the need for more complex preparations. These are challenging issues, but the history of RNAi treatment over the past 20 years has proven the power of perseverance. With the development of many innovative technologies in excipients and payloads, there is no doubt that there will be more breakthroughs in the future. (Sina Pharmaceutical Compilation/newborn)
Reference source: The current state and future directions of RNAi-based therapeutics
Offer Medical Cord Clamp,Medical Use Clamp,Sterilized Umbilical Cord Clamp From China Factory .China Medical Cord Clamp,Medical Use Clamp factory, visit here to find the Sterilized Umbilical Cord Clamp,Pe Material Umbilical Cord Clamp that you are searching for.Medical Cord Clamp,Medical Use Clamp,Sterilized Umbilical Cord Clamp,Pe Material Umbilical Cord Clamp
Medical Cord Clamp,Medical Use Clamp,Sterilized Umbilical Cord Clamp,Pe Material Umbilical Cord Clamp
Luck Medical Consumables Co.,LIMITED , https://www.luckmedical.com
