Recent review of the medical industry: Huada Gene Prospectus attracted 59 questions from the CSRC
Diabetes is the number one chronic disease in China. According to the statistics of the International Diabetes Federation, in 2015, China's diabetes patients ranked first in the world, reaching 110 million, of which more than 1 million people died of diabetes every year. In order to better promote the sustainable development of China's major health industry, Johnson & Johnson Medical and Jingdong joint strategy to achieve strong cooperation, hoping to debut the newest blood glucose meter as an opportunity to work together to promote diabetes management based on big data, in-depth exploration of the future of mobile health A new model of development. In addition, what are the hot spots in the medical industry in the near future? The editor of OFweek Medical Network will take you to review it.
News review
Johnson & Johnson and Jingdong Pharmaceutical launch new full-color intelligent blood glucose meter
Recently, global family blood sugar management expert Johnson & Johnson Medical announced that it has teamed up with JD.com to achieve strategic cooperation and jointly promote the exploration of chronic disease management such as diabetes based on big data. As the first step of this cooperation, Johnson & Johnson's first full-color smart home blood glucose meter - Johnson & Johnson, Stable and Smart, was launched in Jingdong today. Mr. Amechi Nwachuku, Vice President of Johnson & Johnson Medical Europe, Middle East Africa and Asia Pacific, said that “Johnson Medical has been actively involved in chronic disease management and mobile healthcare. Jingdong Pharmaceutical Health is a leading retail channel in China's pharmaceutical health, with strong brand influence and quality. The customer group has also actively explored the management of chronic diseases, which coincides with the development strategy of Johnson & Johnson Medical. We are very pleased to join hands with JD, to explore cooperation in diabetes management and even mobile medicine, and jointly promote China. The development of chronic disease management." [ Original reading ]

The road to listing of Huada Gene has been twisted and twisted: the prospectus has attracted 59 questions from the CSRC.
In December 2015, after the first time the Huada Gene submitted the prospectus to the China Securities Regulatory Commission, the review was suspended due to the incomplete application documents. On March 14, 2017, Huada Gene updated the listing prospectus, compared with the previous submission. The number of pages has increased from 523 to 607 pages. The latest contents include the latest financial data, changes in the shareholding structure, employee compensation description, new business expansion, and future prospects. They are described in detail. Pure, only owe the wind. However, whoever thought, followed by the CSRC to issue a letter to Huada Gene's prospectus, asked a series of 59 questions.
In the feedback from the China Securities Regulatory Commission, issues such as the independence of the company and the eligibility of institutional shareholders were highlighted, and “whether the Huada Gene itself is independent of the controlling shareholder†and “the compliance of the 43 institutional shareholders’ shares is in complianceâ€, Issues such as "why are customers mostly research institutions" have become the focus of regulatory attention. [ Original reading ]

Samsung developed a professional wearable medical device
On March 20, 2017, we all know that Samsung's most representative product in the smart wear field is the Gear series of smart watches, but everyone knows that Samsung originally planned to launch a professional medical wearable device Simband.
Simband is said to have a modular design that tracks blood pressure, skin temperature, heart rate and sweat, as well as sharing it with your doctor in real time via the platform. It seems that this device is in line with the current trend in the field of smart wearable devices, that is, health.
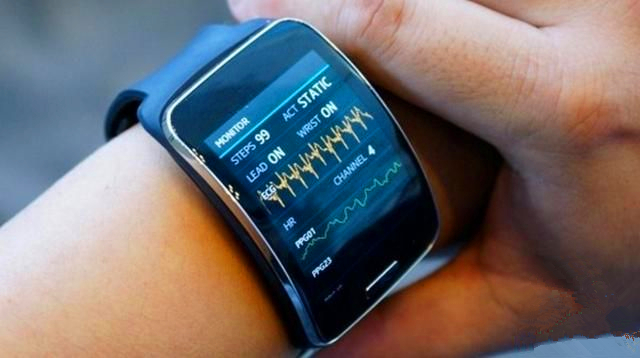
But unfortunately, Simband has not been available yet. Samsung said it is mainly subject to regulatory, research and development and cost factors, and is not suitable for sale to ordinary consumers. Because everyone is accustomed to the relatively low-priced bracelet or watch products, and high-end medical wearable devices such as Simband, there is no best time to market. [ Original reading ]
Reflective Sheeting,3M Reflective Sheeting,Best Reflective Tape,Colored Reflective Tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytapes.com