90% of rare diseases have no cure, can we do with genetic editing?
Author: Yang Hui Release Date: 2019-06-05
Before I came here, I only knew that one was focused on science and humanities. I just heard that there is still a daydream. I also fooled several of my students for daydreaming. Our dream is very simple. I want to make a rare drug and hand it to the patient, so that we won’t help when we receive a message from the patient. .
I have just said that there are 7,000 rare diseases, and we may not be able to do it in our lifetime. In fact, we still have a bigger daydream, that is, we hope to build a platform for the development of rare disease drugs, so that more people can dream with us. I hope that when we wake up, we are not 90% of rare diseases without drugs. I hope that most rare diseases can have their own corresponding genetic drugs.
How far is the genetic drug of rare diseases from us?
Hello everyone, I am Yang Hui, from the Institute of Neuroscience of the Chinese Academy of Sciences. My research direction is mainly genetic editing.
Gene editing can be said to be one of the most important technologies in life sciences in the 21st century. We can use it to improve the excellent traits of plants and animals, as well as to control pests and diseases, and to treat diseases. Some scientists are imagining the combination of cloning techniques to resurrect some extinct creatures, such as mammoths.
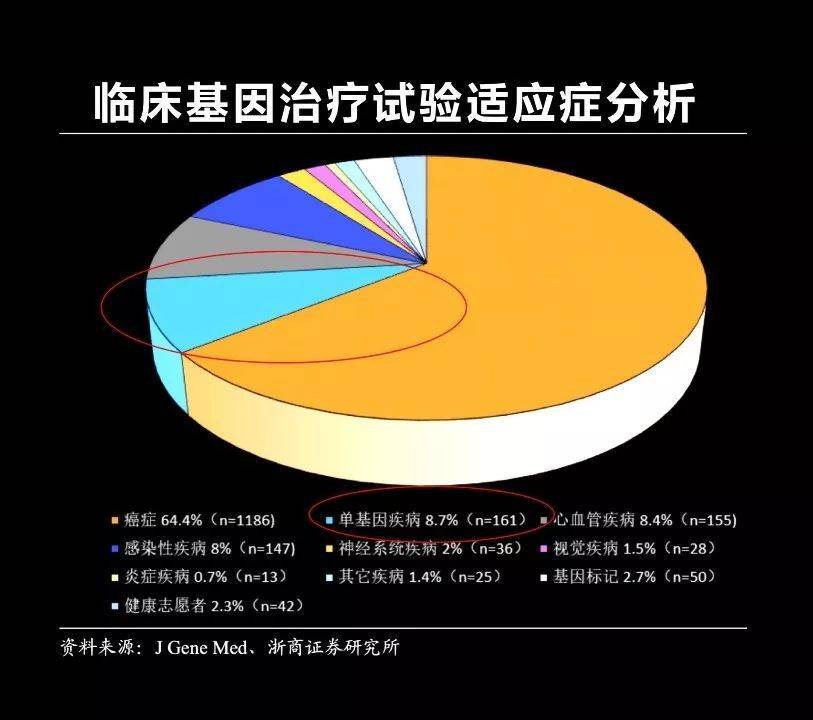
Gene editing technology, in fact, the most important use is to treat a variety of diseases, including cancer, cardiovascular disease, Alzheimer's disease, and some infectious diseases. Today, I mainly tell you about one of the most important aspects of genetic editing, how to treat a single genetic hereditary rare disease.
Genes must be known to everyone, that is, DNA fragments with genetic effects. Our DNA consists of more than 3.1 billion base pairs, which are composed of four organic bases of ATCG.

We probably have 20,000 to 25,000 genes. Although everyone is very different, even though I am compared to Einstein, the genomes are very similar, less than 1% difference. Einstein and chimpanzee, guess how big the difference? In fact, it is a little more than 1%, but Einstein has 200 IQs, and Chimpanzee IQ is about 70, which is about the equivalent of a child of seven or eight.
So any small changes in the genes may change our major functional traits. In an extreme case, even a change in base can lead to changes or deletions in gene function, leading to disease. And almost 50% of our single-gene genetic diseases are caused by a single base change.
So the scientists thought, if you can have a scorpion to replace this base, so that you can achieve the purpose of disease treatment. The idea is very simple, but it is very, very difficult to implement. why?
First of all, the DNA is very small, only 2 nanometers, 200 times thinner than the hair, so it is very difficult to operate with a pair of tweezers. There is only one base change, but our genome has 3.1 billion base pairs. It is very difficult to find it, and will it cause another 3.1 billion bases to be destroyed? It is also impossible to know.

So scientists have been pursuing a method that we call genetic editing. Gene editing is the insertion, knock-out or replacement of small pieces of DNA in a particular fragment.
There are three processes to achieve this. The first is to identify, need to find it, find a specific location, a specific mutation, which is the most critical part. After finding this mutation, the mutation should be cut off. After cutting it off, provide a normal template and fix it.
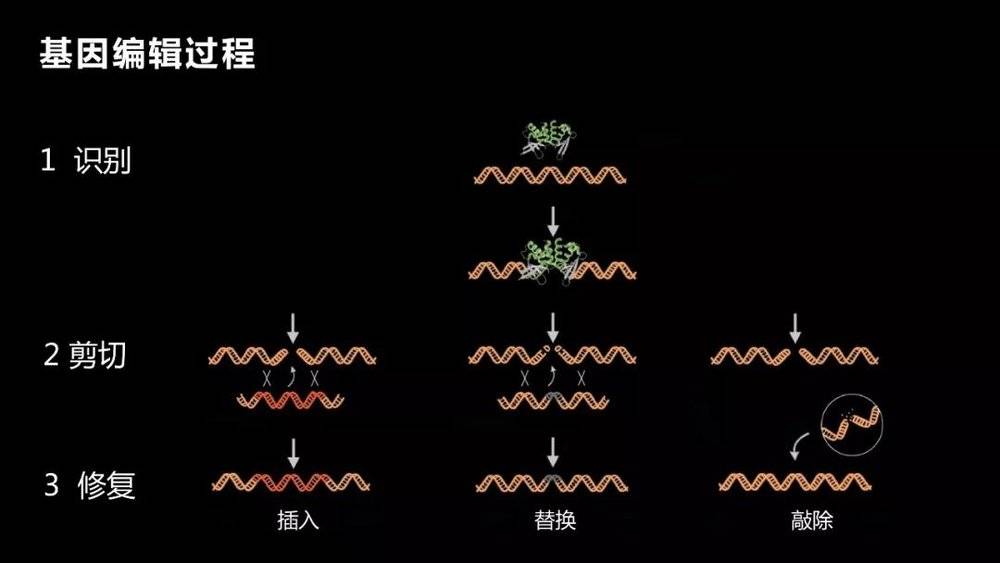
The first process, finding this particular location, is very critical and very difficult. The key is to change only the genes that you want to transform, not to destroy other genes, or not to achieve the purpose, but may cause many adverse consequences, just said, a base change may also cause fatal harm. Why is it very difficult to say? As you can imagine, you can choose to edit one or a few bases in the 3 billion bases. It is not easy to find them. Even if you have a house number, you have to find it for a long time.
So my boss, MIT's Rudolf Jaenisch, can't wait. During his post-Bao period, in the 1970s, when he was only about 30 years old, and I was about the same size now, there was a whimsical idea, not directly looking for the house number, first to change the genome.
But the individual of the animal has many cells, how to achieve many cell DNA changes? It happened that the test-tube baby had just appeared, so he thought that if the fertilized egg was directly changed, and then the embryo eventually developed into an individual, would all of its cells carry mutations in this gene? He thought so and did it.

He injected the DNA of the virus directly into the fertilized egg and then moved it into the mouse mother. After three weeks, he obtained the mouse and performed genetic identification. It was found that all the cells of the mouse contained the DNA of the virus, and the DNA could be passed on, and the progeny also carried the DNA.
Rudolf Jaenisch was named a member of the American Academy of Sciences for this discovery and was received by the President. But unfortunately, this technology missed the Nobel Prize. why? We can see that the most critical thing is that the way the virus is inserted into the genome is a random insertion, not a fixed point insertion. Randomness can cause damage and has not achieved the best results. In a strict sense, it is not a gene editor.
After more than a decade, two other scientists actually achieved the genetic editing of the fixed point , and thus won the 2007 Nobel Prize.
After more than 20 years, the gene editing technology has evolved over several generations until the third generation in 2012. After we call CRISRP-Cas9 technology , gene editing becomes a technology that almost every laboratory can operate.
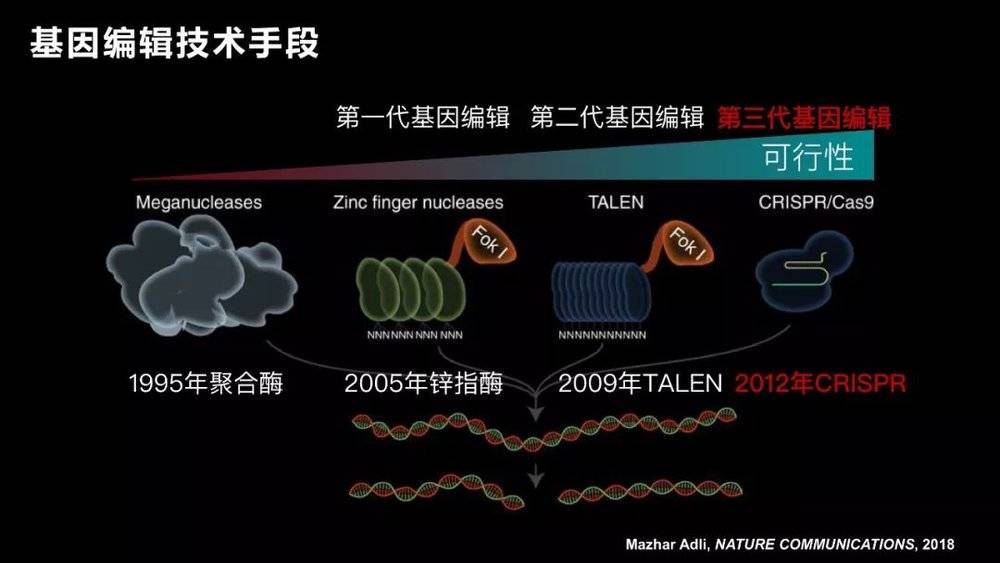
The advantage of CRISRP-Cas9 is that it is simple and efficient. By attaching a label, it operates on one gene, and two or three can be used to achieve multi-site operation. So since its report, many scientists at home and abroad have quickly followed up. Professor George Church of Harvard and Professor Zhang Feng of MIT are undoubtedly the winners of this competition. They applied this technology to mammalian cells for the first time.
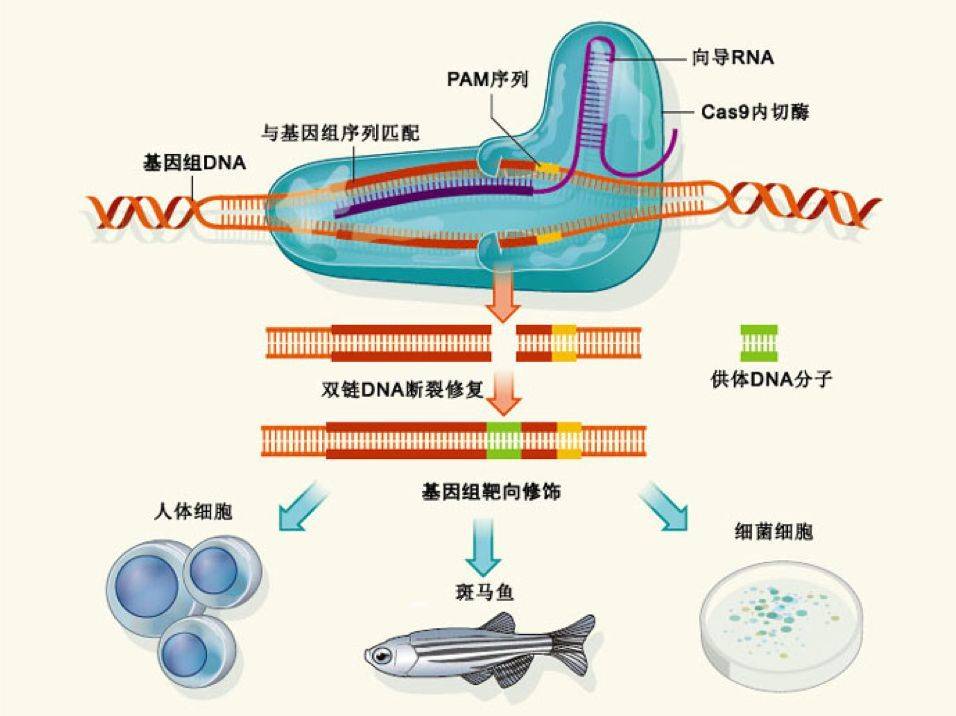
I personally and genetically edited it very well. As early as the Ph.D., I used the most classical homologous recombination gene targeting method to construct a variety of genetically modified mice, albeit very complicated.
I was probably in the United States after the end of 2012. After one month, Zhang Feng and George Church published the results of CRISRP-Cas9, when Zhang Feng was in the laboratory next door. Fortunately, the same post in China, the same post from China, called Wang Yiyi, he mainly studied the second generation of gene editing technology, and we both hit it off. The discovered CRISRP-Cas9 only proves its role in cells. Can it be used efficiently in genetically edited animals? At that time everyone did not know.
The two of us went to the Zhang Feng laboratory next door to bring the plasmid to the next day. The CRISRP-Cas9 system was injected into the fertilized egg of the mouse by microinjection and then transferred to the mouse.
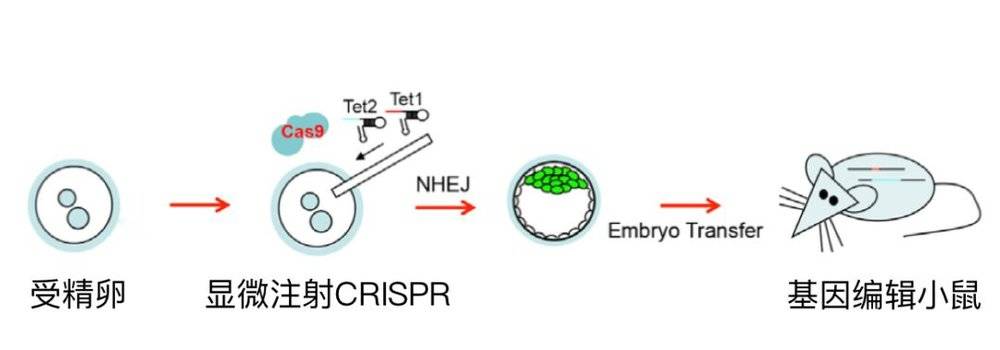
Therefore, within a short period of six months, we have proved that CRISRP-Cas9 can efficiently obtain a variety of genetically modified animals, including knockout mice, knock-in mice, and precisely repaired mice. The results are also published. Above the top magazine in the biological field, Cell.
A more interesting episode is that because students in mainland China may work harder, going abroad often follows the principle of 996 - probably longer than 996. Foreign Boss can usually operate up to 100 to 200 embryos per day. You can look at this micromanipulated embryo. We operate one by one.
But I was doing very fast at the time, and I could do more than 400 embryos a day. My tutor Rudolf Jaenisch felt a little surprised. I ran to the lab the next day to see me in the experiment. After reading it, he was convinced. .
After returning home, my partner Wang Yiyi went to the Animal Institute of the Chinese Academy of Sciences in Beijing to apply for CAR-T tumor immunotherapy, and I continued to use genetic editing technology to make a variety of animals. This has changed from a mouse to a monkey. Why do you make a monkey? Because monkey brain development and advanced cognitive behavior are unique to all model animals. If we use gene editing technology to make a variety of genetic tool monkeys, it will help us understand the brain and help with the treatment of some brain diseases.
Just a year after our lab was set up and we were preparing to produce a variety of genetically edited monkeys, an accidental discovery completely changed the direction of our lab at the time.
We found that CRISRP-Cas9 can not only edit a single gene . The mouse has 20 pairs of chromosomes, which is 40 chromosomes. If one label is attached to one chromosome, CRISRP-Cas9 edits one gene. If 10 labels are attached, it edits 10 genes. When we put 100 labels, we found that it was not editing 100 genes, but the entire chromosome disappeared.
We found this very exciting at the time. Can this be applied to the treatment of Down's disease? Everyone knows that Down is also known as Trisomy 21, which has a chromosome 21 more than normal. If we have a label on the 21st chromosome, can we effectively eliminate this chromosome and achieve disease treatment? What is the purpose?
To test this idea, we induced pluripotent stem cells from Down's patients. There are three chromosomes 21 in this stem cell. We introduced CRISRP-Cas9 and found that it can efficiently and specifically eliminate this extra chromosome 21.

After the publication of relevant results, it received the attention of many media and high praise from international counterparts, saying that our technology can provide a new way for the treatment of Down's disease.
I thought that the story has already ended here, and then continue our previous research. But the big thing that touched me was that the media just reported that I received many emails from the families of Down patients. One of them was to encourage us to continue the research, and the other was to ask when this method could be clinical.
Some parents are willing to contribute generously to sponsor this study. Although this is very common abroad, it is very rare in China. This is more impressing me, so personally, I began to pay more attention to this group of rare diseases.
Rare diseases are not uncommon. There are more than 7,000 rare diseases found in the world, with a total population of more than 300 million, more than the total population of cancer . Most of them are single-gene genetic diseases, and half of them occur in childhood. Once the disease is accompanied by life, it will bring pain throughout life.

Down's syndrome is one of the most common newborn babies in our country. About 20,000 to 30,000 Tang babies are born each year in China. There are also more famous gradual freezing diseases like Hawking, and our familiar hemophilia and albinism are rare diseases.
More than 90% of these diseases do not have any treatment. This situation is particularly evident in China, where 21 rare diseases are faced with drugs abroad but no drugs in China.
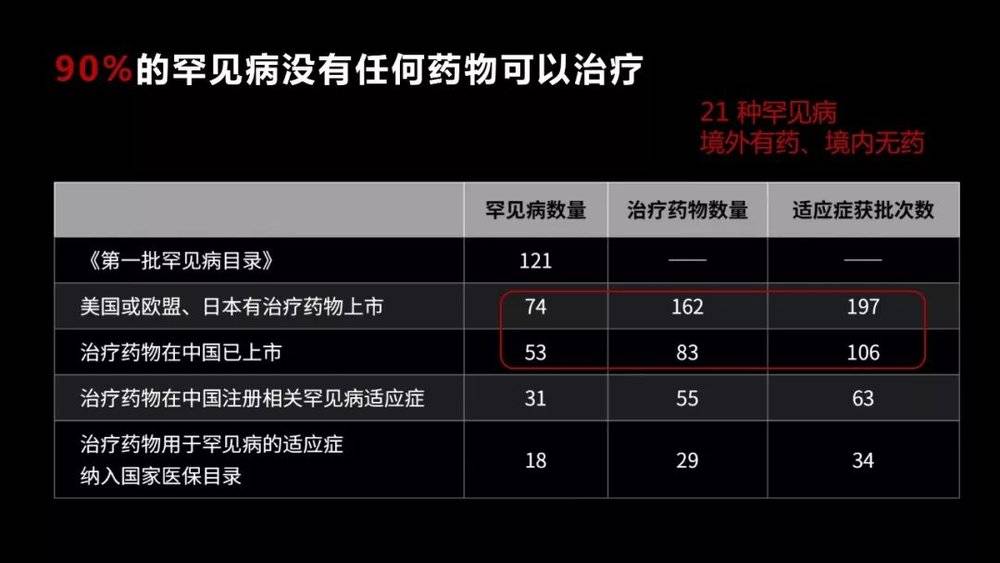
Why are there so few drugs for rare diseases? There are two main reasons. One is that although the total population of rare diseases is relatively large, it has a very small population for each disease .
Any new drug research and development needs about 2 billion to 3 billion US dollars of investment, which is very huge, even if the generic drug is 200 million to 300 million US dollars. Therefore, large companies generally want to develop drugs for common diseases, so once they are developed, the return will be very high. Any rare disease is equivalent to an original new drug, its cost is very high, and the income is very small, so many big companies are not willing to study it.

The other one, even if some drugs are researched, its therapeutic effect is very limited . Many diseases require medication for life. And as the disease progresses, the effects of the drug are getting worse and worse, and the patient is getting more and more harm. Whether it is for the patient or for the society, it has brought a huge burden.
Because 80% of rare diseases are monogenic genetic diseases, scientists actually proposed in the 1970s whether treatment can be achieved by gene therapy, and the concept of genetic drugs came into being. In those days, gene drugs introduced genes with normal foreign genes into cells with gene defects in various ways to achieve functional recovery and disease healing.
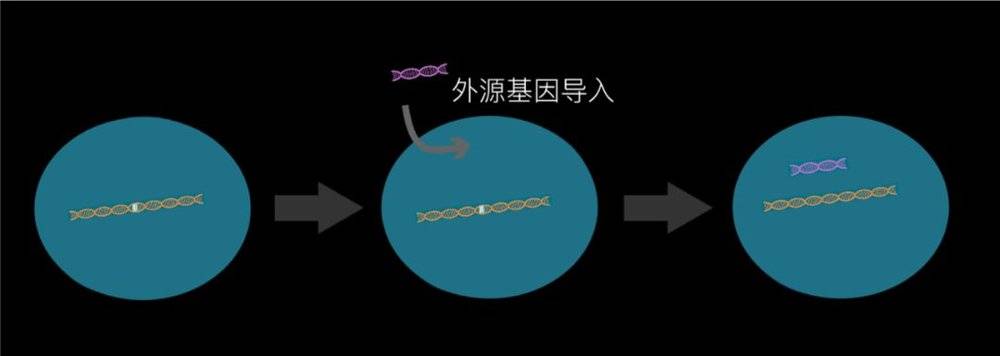
But because it is a foreign gene, unlike its own genes, it will be lost and degraded as the cell divides, unstable, and the effect can often be several weeks to several months. Even the most mature and most commonly used way to import viruses can only last a few years. It may be enough for two or three years for the mice we experimented with. But patients are often sick during childhood, and they need drugs that are effective for decades.
With the growing maturity of genetic editing methods, scientists seem to have found a perfect solution to directly repair endogenous genes through CRISRP-Cas9 technology, achieving a once -in-a-lifetime effect.
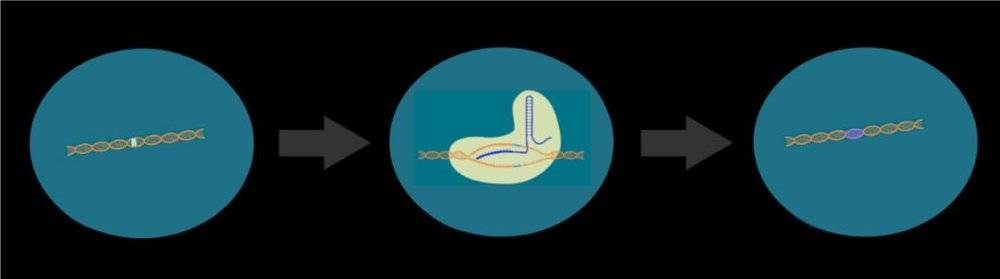
For example, if a piece of clothing breaks a hole, you can make a patch that seems to be very simple and efficient, but it affects both aesthetics and comfort. The way of gene editing is equivalent to accurately stitching with scissors and needles to achieve the effect of in situ repair.
So the concept of the current gene drug has been greatly expanded compared to decades ago. Any means of regulating genes can be called gene drugs, including gene editing, as well as antisense nucleotides and genes. Expression, etc.
Next, I will introduce the development process of the whole gene drug and how the gene editing technology can be applied to the research and development of rare disease gene drugs.
We take spinal muscular dystrophy (SMA) as an example, which is a disease that our laboratory is studying. Its incidence is one in ten thousand, and it is already a relatively common disease in rare diseases.

It is an autosomal recessive disorder caused by a defect in the SMN1 gene. The carrying rate of this gene is very high. One person carries it every 50 people. If both parents are carriers, the probability of a child's illness is about 25%.
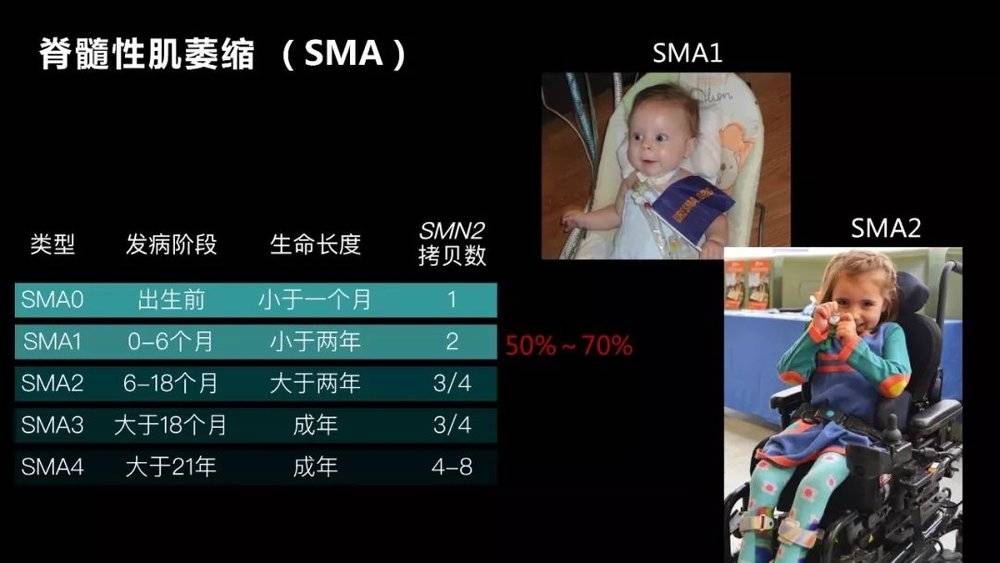
Since the defect of this gene causes apoptosis of motor neurons, most children born after the disease are over two years old. Even if some survive, the athletic ability is greatly impaired.
Since the discovery of this disease-causing gene, it has been 21 years since the first drug of the disease was finally launched. Why is it so long?
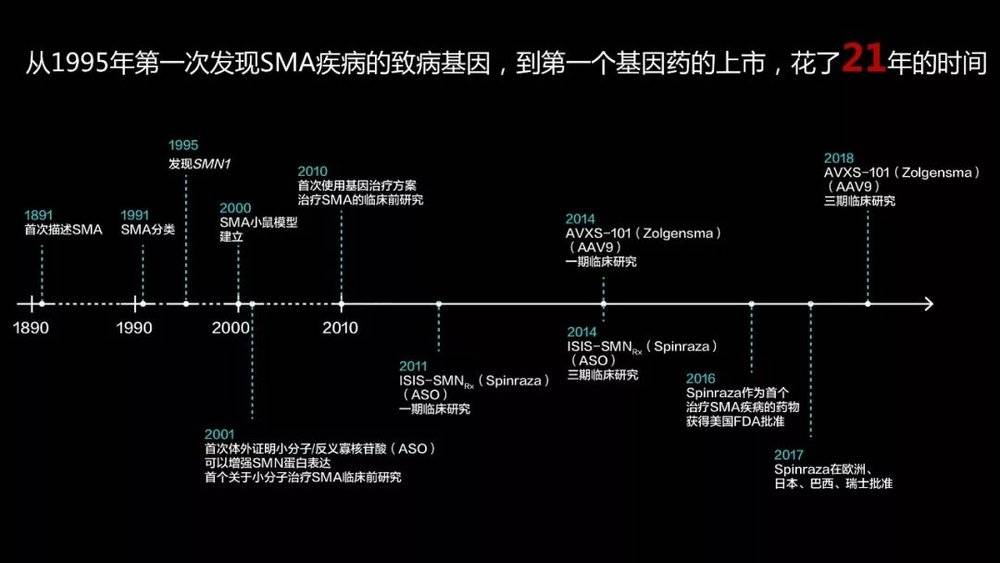
In general, we study single-gene disease as this routine. First, after humans discovered the disease, they hope to reproduce the disease in mice through gene editing technology.
How to do it? It is to knock out a SMN1 homologous gene by gene editing technology to obtain a disease-bearing mouse. Scientists have found that mice do as a diseased person, motor neuron apoptosis, and die within three weeks.
After getting this animal model, gene therapy actually went about a third. After that, scientists will think of various ways of intervention (antisense nucleotides, gene overexpression, gene editing and repair, etc.) to restore the expression of this gene to achieve the purpose of curing mice.
After many years of hard work by many scientists, I finally got a very good therapeutic effect on mice. You can then start applying for a clinical trial. After the safety evaluation of large animals, after a long period of Phase II and Phase III experiments, the final drug was marketed.
As you can see, the drugs for rare diseases, from the beginning of clinical to the final listing, are only a short three to five years. The drugs for our common diseases take about 10 to 15 years.
Why is it shortening so much? In fact, both the US FDA and the Chinese CFDA have green channels for rare diseases. Because there are fewer patients with this disease, in order to save costs, once it has effect on a small number of patients, it will be conditionally listed.
This drug was probably listed at the end of 2016. Before the IPO, I heard from a cooperative doctor that his family members were cheering and their children were finally saved. But in fact, I was not happy for too long. When the price of this drug came out, the family members were silent.
This drug requires about 800,000 RMB for one injection, and it takes two to three injections a year. Once injected, it needs a lifetime injection. This is not something that ordinary Chinese families can afford.
A better way to treat a gene is through virus-mediated overexpression of the gene. For spinal muscular dystrophy, it claims to last two to five years, but the cost is even higher, about $4 million. And once it enters the body, it is produced by a virus. If the second injection, it will cause a great immune response. Therefore, virus-mediated gene drugs are generally one-off.
So we are thinking, is there a simple and efficient way, or a once-and-for-all way to treat? That is, genetic editing. Our laboratory successfully used gene editing to achieve repair of SMA disease genes in mouse animal models. After the repair, we can see that the weight of the mice is the same as normal. The normal sick mice die within three weeks, and our mice have been observed for more than one year and are very healthy.
And the way this treatment is different from other gene treatments, there is no attenuation, so we are considering how to push this technology to the clinic.
It is not so easy to promote the treatment of rare diseases in China.
The first bottleneck is the lack of core patents for gene editing technology .
As you can see, there are about 7 kinds of rare disease gene drugs that have been listed all over the world. Seven of them are antisense nucleotides or virus-mediated overexpression of these traditional gene therapy methods. All of them are produced by foreign companies, and all the basic patents of these gene therapy technologies are in the hands of foreign companies.
So far, although there are no genetically-edited drugs on the market, there are already two clinically edited drugs, all of which are produced by American companies. From the discovery of gene editing technology in 2013 to the clinical, in fact, only a short five years, the development is very very fast.
The core patents for gene editing technology, especially the earliest batch of basic core patents, are also applied by European and American scientists, and the patents are also in the hands of their companies. If we want to study the drugs of genetic editing, and apply the basic technology, we must bear the high patent fees.
Another bottleneck is the imperfection of China's gene therapy industry . Although gene editing technology is very new, the concept of gene therapy has actually been done abroad for decades, so all drugs are produced abroad.
But China's industry has just begun, so even if our basic research is close to the level of European and American scientists, a very big bottleneck is the transformation of industrialization, how to produce clinical-grade viruses, and bring these genetic editing tools into the field. Into the human body.
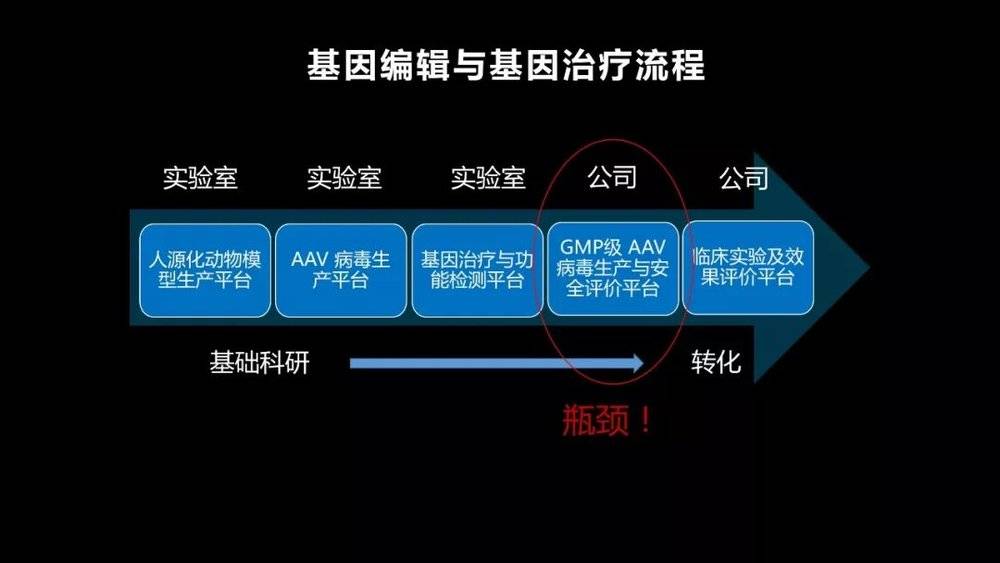
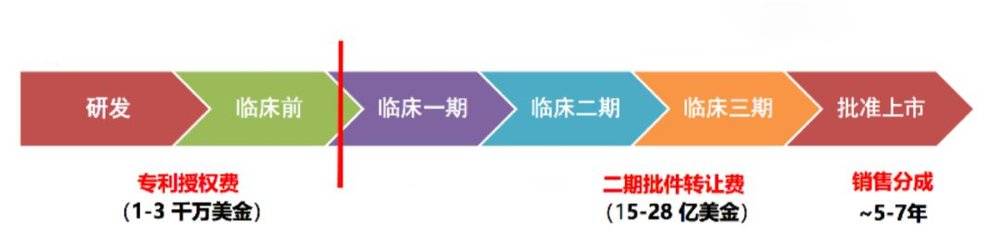
As you can see, basic scientific research and pre-clinical research, in fact, its real economic value is not big. But once we can break through this bottleneck and get good results in the first and second phase of clinical trials, its value will increase by a hundredfold. In the future, the problem facing the entire gene therapy industry in China is to strive to break through this bottleneck as soon as possible, and to transform our knowledge of basic research into clinical practice as soon as possible.
The third bottleneck is the problem of genetic editing technology itself . Why gene editing technology has been considered by everyone to be the best way, but so far only two drugs are clinically available? In fact, the most critical point is its insecurity, which is the off-target effect of genetic editing.
As you can imagine, there is only one target site, but there are 3.1 billion off-target locations, so the probability of off-target is very, very high. If off-target occurs on an oncogene or tumor suppressor gene, it can lead to cancer.
How to effectively avoid off-target is one aspect, and the other is how to detect these off-targets with highly sensitive and sensitive techniques. Our laboratory has recently established a method for efficient and sensitive detection of off-targets across the genome. We have found that one of the most widely used single-base editing techniques has a large number of off-targets, so its application in clinical is not safe.
The discovery was published in the journal Science. After the publication, two companies that were pushing the clinical project were forced to terminate. As you can imagine, once they have been clinically ill, they will cause a lot of off-targets on the cells, which may benefit early in the patient, but may become a high-risk population after a few years.
Just a few days ago, Nature magazine reported that the results of our recent research team also found that single-base editing technology can not only cause DNA off-target, but also cause off-target of RNA, further indicating that the technology is actually It is very imperfect.
We don't have to be pessimistic. In fact, on the other hand, this also gives us China, whether it is a scientist or a business, an opportunity. What is the chance? That is, we are able to establish new standards in the industry and develop more efficient and safer genetic editing tools. If we can win in this competition, we are likely to break the blockade of the core patents of genetically modified scientists in Europe and the United States.
Before I came here, I only knew that one was focused on science and humanities. I just heard that there is still a daydream. I also fooled several of my students for daydreaming. Our dream is very simple. I want to make a rare drug and hand it to the patient, so that we won’t receive a message from the patient. Love can help.
I have just said that there are 7,000 rare diseases. Maybe we can't finish it in our whole life. In fact, we still have a bigger daydream. We hope to build a platform for the development of rare diseases and let more people join us. dream. I hope that when we wake up, we are not 90% of rare diseases without drugs. I hope that most rare diseases can have their own corresponding genetic drugs.
Finally, genetic editing techniques can have very bad effects if used in bad ways. Gene editing infant incidents, whether at home or abroad, in the scientific community, society or the government, have a common view, that is, resolutely oppose the occurrence of such incidents.

So the gene editing tool itself is not wrong, and what our scientists are doing is to make the tool sharper and sharper, making it more efficient and accurate. Whether it is an angel or a devil is the choice of all of us.
Source: WeChat public number: one seat (ID: yixiclub)
Our company specializes in providing Nootropics raw materials -Nootropics Nootropics Nootropics powder Nootropic Active Pharmaceutical Ingredients Nootropics peptides
We also provide Active Pharmaceutical Ingredients // Bodybuilding Peptide //Sarms and so on

Nootropics , Nootropics powder , Nootropic Active Pharmaceutical Ingredients , Nootropics peptides
XI AN RHINE BIOLOGICAL TECHNOLOGY CO.,LTD , https://www.rhinebiotech.com
