The global drug price has cooled down, and the digital innovation of Takeda Pharmaceutical has been turned over.
Takeda Pharmaceuticals is the largest pharmaceutical company in Asia. In 2017, after the acquisition of "Snake Swallow", the market value surpassed Gilead and became a top10 pharmaceutical company. Why does Takeda Pharmaceutical sing all the way? Digital health may be the focus of Takeda Pharmaceutical's next 10 years of development.
Takeda Pharmaceutical CEO has said that the future positioning of Takeda Pharmaceutical is to become a leader in digital health in 2025.
The arterial network found that the digital innovation was equally important for Takeda and the acquisition of Charles by combing the motivation and reason for the digital innovation of Takeda Pharmaceutical. Takeda's digital innovation defense line is mainly to consolidate the leading position in the existing main disease field.
Takeda Pharmaceutical: From me too to me better
Made in Japan has always been considered a guarantee of quality and has a good reputation. Japan has been reborn as a manufacturing power from the ruins of World War II. The secret lies in the imitation and transcendence. After World War II, Japan took advantage of its policies and made a large number of counterfeit US products. However, Japan is not satisfied with the American products of the cottage, but disassembles the products, learns the design concept, and carries out independent innovation on the basis of imitation. This project was defined by the Japanese government as "reverse engineering."
This "takenism" is also the reason for the growth of Takeda Pharmaceutical. From 1950 to 1970, with the support of Japanese government policy, Takeda Pharmaceutical introduced patent technology, updated and improved patents, and formed its own small patent. In the following ten years, Takeda Pharmaceuticals began to move into the global market from the “Me-too†imitation products of the 1980s to the “Me-better†imitation products of the 1990s. It is worth mentioning that since the 1970s, Takeda Pharmaceutical's R&D investment has remained at 20%-25% of sales. This is not a low value in the pharmaceutical industry.
The benefits that the policy brings to Takeda Pharmaceutical are not limited. In the 1960s, Japan implemented a health insurance system that reduced the medical expenses that patients had to pay, and doctors could benefit from the sales of prescription drugs. In the same period, the Japanese government also set up a high-price, strict review system to prevent overseas drugs from entering the market. In order to sell drugs in Japan, overseas pharmaceutical companies must be represented by local pharmaceutical companies. With the government opening the way to remove obstacles, Japanese pharmaceutical companies have invested more in research and development, laying the foundation for later overseas expansion and product development.
Many times, the flowers grown in such a greenhouse cannot withstand the wind and rain, and the nest is not a skill. However, Takeda Pharmaceuticals is the largest pharmaceutical company in Asia and the only Asian pharmaceutical company to rank among the top10 in the world. Takeda Pharmaceutical has also embarked on an excellent path to localization.
Takeda started distributing drugs to Europe as early as 1978. At the end of the 20th century, Takeda Pharmaceutical established R&D centers in the United Kingdom, the United States, Ireland, and the European continent. Affected by Japanese drug control fees, Japan joined ICH in 1997, completely opening up the pharmaceutical market, and Takeda is also in urgent need of reliance on the local market. Takeda Pharmaceutical has cooperated with companies such as Abbott, GSK, Novo Nordisk and Eli Lilly to develop drugs to break through the FDA's listed drugs.
According to Takeda's fiscal year 2017 data, sales contributed by the Japanese market accounted for only 32.8% of Takeda's revenue. Considering Takeda's acquisition of Charles in 2018, Takeda's dependence on the local market is expected to fall below 20%.
Digital innovation to solve the dilemma of pharmaceutical companies
Looking at the world, many pharmaceutical companies have been reorganized to divest non-core businesses and focus on the pharmaceutical business. For example, Johnson & Johnson sells the diagnostic industry. The CEO of Slan Suk also chose to reorganize the R&D department shortly after taking office. Takeda's acquisition of Shire is also a member of the reorganization of pharmaceutical companies.
It is not unreasonable for pharmaceutical companies to take action. Major pharmaceutical companies have continued to choose two routes. The first is restructuring, which is to acquire new companies; the second is to cooperate with external companies. Reorganization is a group warming, and cooperation with digital innovation companies is a match that shines in the dark.
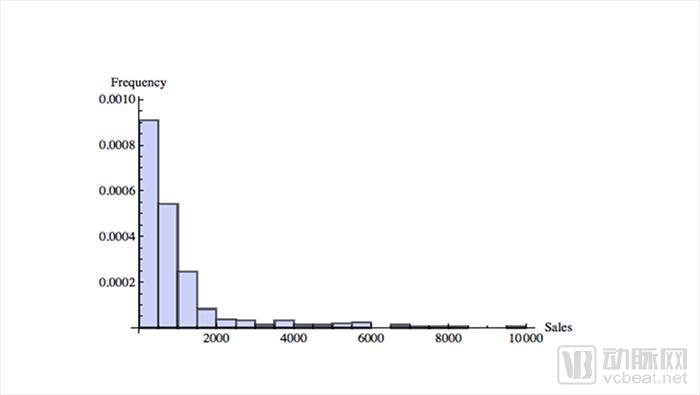
2012 drug sales data
The sales of medicines are randomly distributed. The upper graph shows the sales from low to high in the X-axis, and the Y-axis indicates the sales volume of the corresponding drugs. The farther to the right, the greater the sales of medicines. From the chart we can see that very few drugs account for a large amount of sales. This distribution is almost the same in any year of drug sales. For example, oncology drugs, data from IQVIA show that the top 35 drugs account for 80% of total expenditure.
The challenge for pharmaceutical companies lies in these “long tail†distributed drugs. Some rare blockbuster products determine the productivity of a company and the entire industry. For example, Gilead and Amgen.
In contrast, most mature companies are increasing in a near-normal distribution, with relatively similar products clustered around averages that do not produce very high or very low sales. So the production results are usually predictable. Product improvements only need to be moderately updated, and there are careful plans and rules to implement.
The above picture also shows that the patent expiration of key drugs can cause devastating blows to drug companies. Although Takeda is ambitious in its globalization strategy, it has to face the pressures of all parties.
The first is financial pressure. The sales of the popular lansoprazole, pantoprazole and candesartan patents have expired. The sales of Takeda have not increased as fast as before 2012. In 2016, there was a decline, equivalent to the US dollar. Sales were approximately $16 billion. The turnover in fiscal 2017 was $16.7 billion, and no strong growth was achieved.
On the policy front, due to the aging population and the shadow of control fees, the Japanese government has proposed that the sales volume of generic drugs will reach 70% in 2017 and 80% between 2018 and 2020. If the drug company does not respond in time, it may not be able to bear the burden of policy changes and fall into a trough.
Past performance is not predictive of future success, and this sentence is very applicable to pharmaceutical companies. So how to solve it? According to the figure, the product production can be concentrated in a certain product pipeline to establish a dominant position in a certain field. This road is undoubtedly a self-digging grave, because the pharmaceutical company will only walk into the dead end of relying on a single product.
Another way is that the pharmaceutical company is already using the restructuring, allowing itself to focus on the main products while extending the product line. Takeda Pharmaceutical's acquisition of Charles is in line with this intention. Through the acquisition of Charles, the completion of global restructuring, Takeda will focus its business resources on core businesses such as cancer, digestive organs and central nervous system, and will divest non-core businesses. Takeda also hopes to improve the financial situation due to the acquisition of Charles. Shire, which has made great achievements in the field of medicine in the field of rare diseases, can also expand Takeda's product line.
Takeda Pharmaceutical CEO Christophe Weber said: "The field of Takeda Pharmaceutical's focus on digestion, oncology, neuroscience, etc. After the acquisition of Charles, we will add another important area of ​​rare diseases."
Takeda Pharmaceutical also said that the acquisition of Charles can save at least $600 million in research and development costs. Reducing research and development costs and increasing drug diversity have become the main needs of Takeda Pharmaceutical.
In addition to restructuring and acquisitions, the other is decentralization, choosing smaller and more focused innovative companies to collaborate, and digital health companies are taking the lead in all areas of research and development, sales, and prognosis, and pharmaceutical companies can choose to invest. M&A, cooperation and other methods form an innovative ecosystem with digital health companies to reduce risks and enhance diversity.
Pharmaceutical companies are adopting a two-pronged strategy to internally focus on narrowing the therapeutic areas, targets, therapeutic mechanisms and compound types in drug development, which may increase the success rate of drug development. On the other hand, it is working with external companies to enhance external R&D capabilities.
These two strategies are complementary, and external cooperation can compensate for the diversity reduction and risk brought by internal focus. That is to say, as a big tree, the pharmaceutical company builds the excess branches of the main body and guarantees the quality of the results. Pharmaceutical companies also need to support some wildflowers and weeds at their feet, because these wildflowers and weeds can bring more diversity.
Takeda Pharmaceutical Digital Innovation: Consolidate the Status of Existing Diseases
After understanding the reasons for digital innovation, we need to know the direction of digital innovation of Takeda Pharmaceutical. If Takeda Pharmaceutical wants to stabilize the position of top20 pharmaceutical companies in the world, it must first launch a batch of products that can't stand the test. Therefore, Takeda needs to concentrate enough on several pharmaceutical fields. The digital innovation of Takeda is also to enhance the R&D and therapeutic power in the field of diseases that Takeda is the mainstay.
Takeda did not adopt the model of giant pharmaceutical companies to carry out digital innovation, but it is more focused on digital innovation projects that can solve the urgent needs and consolidate the status of rivers and lakes.
Gastrointestinal disease + data
Takeda's best-selling drug, Entyvio (Viduzumab), is used to treat gastrointestinal diseases. Takeda has established a leading position in gastrointestinal diseases. But you can't choose to sit on the mountain. By 2024, the market value of colorectal cancer treatments in the Asia-Pacific market will increase from $5.3 billion in 2017 to $7.9 billion, with a compound annual growth rate of 6%. In digital innovation, Takeda Pharmaceutical began using wearable devices to detect the health of patients with inflammatory bowel disease (IBD).
Takeda collaborates with the Texas Digestive Disease Consultants and the Vanderbilt University Center for Diseases. The pilot project is called iBData. In this project, approximately 100 IBD patients use a special wearable device to track their symptoms and lifestyle factors. These data will be collected and translated into reports to help strengthen patient-doctor interactions to improve the quality of care.
Bruno Villetelle, chief information officer at Takeda, said: “We will continue to face the challenge of how to view and use data, from simple data collection to data aggregation and forecasting. Understanding digital collection and assessment technologies is an important driver of innovation in every part of the healthcare ecosystem. ."
Another company that cooperates with Takeda in gastrointestinal diseases is called Litmus Health. Litmus Health focuses on collecting data on wearable devices for pharmaceutical companies and uses this data to improve drug discovery efficiency in Phase I of clinical trials. Takeda Pharmaceuticals provided funding for Litmus Health's collaboration with the University of Chicago. The test is aimed at the effects of daily activities, sleep and diet on patients with inflammatory bowel disease (IBD).
The value that Litmus Health provides to pharmaceutical companies is revolutionizing data collection methods. Although many patients are now filling out online surveys, it is not much different from traditional paper versions, and patients still need to manually enter data. Litmus Health is able to collect all the different types of smart device data to monitor the patient's vital signs and disease status.
The specific operational flow is that Litmus Health collects data from the clinical trial participants' smart devices, whether they are from Fitbit, Garmin or other smartphones. Litmus Health also needs to integrate this data to standardize the data. The pharmaceutical companies receive a well-organized data report, which allows pharmaceutical companies to directly understand the patient's medication compliance and living conditions.
Collecting patient data is not uncommon, but companies like Litmus Health that focus on pharmaceutical companies are still rare. Daphne Kis, CEO of Litmus Health, believes that their focus on pharmaceutical companies is twofold: First, the cost of existing clinical trials is too high, not a long-term process, and every successful clinical trial means failure. Pay for 9 clinical trials. Second, data analysis companies that pharmaceutical companies need to meet the high standards of FDA data requirements, data companies are more demanding in terms of specialization.
Litmus Health is committed to comply with HIPAA and FDA regulations. Compliance has always been the focus of pharmaceutical companies, and they need to ensure that any data is accepted by the FDA.
Takeda Pharma also launched a patient-integrated solution for Crohn's disease, an inflammatory bowel disease, which provides patients with counseling and rehabilitation advice through digital tools to help patients build confidence. At the same time, Takeda Pharma also collaborated with Boston Digital Venture to globally incubate quality projects in the digital health arena.
Mental illness + patient service
Another main battlefield of Takeda Pharmaceuticals is mental illness, which is the central nervous system disorder. There is currently no effective treatment plan for mental illness and a lack of adequate understanding. Takeda Pharmaceutical hopes that patients with mental disorders will have fewer alternative treatments for people with mental disorders than those with mental disorders such as schizophrenia and depression.
Takeda Pharmaceuticals chose to work with biomarker company Mindstrong Health. Explore together in Alzheimer's disease and refractory depression.
In recent years, neuropsychiatric disorders have been the biggest cause of disability in people under the age of 50, and the cost of mental illness care is the highest among all diseases, partly because of the early onset of mental illness and may lead to disability early. .
For people in the second half of life, neurodegenerative diseases are becoming more and more common, and one in three people over the age of 80 suffer from dementia. Since there are no particularly effective drugs, the most effective way to improve degenerative psychosis is early prediction and intervention.
Takeda Pharmaceuticals works with Mindstrong Health, a biomarker diagnostic company, to explore better health prevention and intervention for mental illness. Mindstrong Health has developed digital biomarkers that collect data from smartphones and continuously monitor brain function. Mindstrong Health's digital phenotypic analysis collects data from smartphones and provides emotional, cognitive, and behavioral measurements.
Digital phenotypic analysis uses three signals from a smartphone, namely the activity, location and social metadata measured by the sensor, such as the number of messages; capture keyboard performance in human-computer interaction, including typing and clicking; using natural language to process the analyzed speech And voice data, this signal provides insight into emotional and cognitive consistency. Together, these signals form the picture of emotion, cognition, and behavior that Mindstrong Health calls a digital phenotype.
Takeda's technology, combined with the powerful Mindstrong Health Platform, will make it possible to apply digital biomarkers to stratify mental health and predict mitigation with new therapeutic interventions.
Cognition Kit
Pharmaceutical companies need to provide beyond pills to better serve patients, especially for people with mental illness. Prevention and post-hospital compliance are also very important. Takeda Pharmaceuticals America Co., Ltd. and Cognition Kit have reached research cooperation and installed special designs on Apple Watch. The app to monitor and assess the cognitive ability of depressed patients to self-mental health status. Regular access to data from everyday life can help with clinical decision making, and healthcare professionals can access patient data and increase patient engagement.
Cognition Kit, a joint venture between Cambridge Cognition Holdings PLC and Ctrl Group Limited, has received widespread attention since its launch of wearable cognitive technology in 2016, the first of its kind with the Cognition Kit. Contract.
Nicke Mowad-Nassar, vice president of Takeda USA, said: "The pilot work aims to establish a large amount of evidence for new methods for measuring mental health outcomes. Technology allows us to establish real-time and objective measurement methods to assess the impact of depression, support patients and clinical Early contact between doctors to change patient care. We are encouraged by the results and look forward to learning more about the combination of technology and healthcare."
In addition, Takeda also held a digital health application contest to treat multiple depression.
AI plus new drug discovery
Digital innovation is always to control the cost of clinical trials to speed up the development of new drugs. Takeda Pharmaceuticals and biopharmaceutical companies and academic institutions around the world are seeking to enhance their in-house drug research and development capabilities through the company's external innovation capabilities. Takeda Pharmaceutical and Numerate collaborate to use AI. Discovered new drug compounds.
Numerate is a software company that developed an algorithm that analyzes large amounts of data and provides Takeda Pharmaceutical with compounds that find new drugs. Numerate is able to use the public data and Takeda's proprietary data to explore new spaces for new drug development. In addition to analyzing large amounts of data, Numerate also has a unique algorithm that optimizes the possibility of designing a combination of compounds in new drug development, ultimately finding high quality compounds.
In the past, individuals could view large amounts of data and perform modeling techniques, but human thinking can handle a limited amount of things. Numerate's technology is unique in that it can identify large data sets and identify opportunities that can be overlooked. In addition to discovering new compounds, Numerate's algorithm can also guide pharmaceutical companies on how to recombine compounds to improve drug performance or discover new properties.
Weitzer said: "We hope that the artificial intelligence platform can use computing power to analyze large amounts of data, enabling Numerate to provide high quality compounds for new treatments that have the potential to meet a large number of unmet needs of patients."
The mode of cooperation between the two parties is mainly that Numerate provides an AI analysis platform, and if new compounds are discovered, the main task of Takeda Pharmaceutical is to promote its commercialization. In general, Takeda needs to spend a year to commercialize a drug.
Incubator project
To establish an innovation ecosystem from the outside, one can work in a cooperative manner, and the second is to select excellent partners through incubation innovation projects. In Japan, Takeda and Whiz Partners set up a joint investment fund to promote the establishment of an innovative ecosystem for drug discovery in Japan. The investment fund will be called the “Drug Discovery Gateway Investment Limited Partnership†(DDG Fund) and will be established in November 2018.
Takeda Pharmaceutical will invest in the DDD Fund in kind in the form of a wholly-owned drug research and development subsidiary Axcelead Drug Discovery Partners and a drug research and development platform company in exchange for limited partner shares. The DDG Fund will approach domestic and foreign financial investors and biopharmaceutical and pharmaceutical industries as limited partners to join the fund. In this way, some academic investments and strategic investments can enable new drug development and support from Axcelead.
Axcelead was previously the research and development department of Takeda Pharmaceuticals, and later became the first integrated drug development solution provider in the Japanese pharmaceutical industry.
Axcelead has all the capabilities associated with non-clinical drug discovery research, as well as people with extensive skills, knowledge and experience in a variety of fields. As a result, Axcelead provides a one-stop solution for innovative healthcare organizations engaged in drug discovery in Japan and other countries to meet their diverse needs. In addition, these services are offered in a wide range of therapeutic areas, from exploratory research and optimization of candidate compounds to the transition to clinical development.
summary:
The case of Takeda Pharmaceuticals tells us that pharmaceutical companies must face digital innovation when they want to develop. Takeda Pharmaceutical's digital innovation combines existing major drugs to provide patients with a more complete solution, demonstrating strong growth through both internal and external repairs.
dental chair accessories,chair accessories parts dental,dental chair spare parts,dental chair parts
Foshan Ja Suo Medical Device Co., LTD , https://www.jasuodental.com
