Effect of room humidity, hydrogen peroxide concentration and condensation factors on the sterilization of vaporized hydrogen peroxide
Literature reading
This article is from the research report of the German Bosch Group and the University of Hohenheim, Germany.
Summary
This study investigated the factors affecting the effect of vaporized hydrogen peroxide on the surface of objects. The article investigated the relative humidity and vaporized hydrogen peroxide concentration, and compared the condensation points of different disinfectants. For the purposes of the study, the scientists developed a series of different H2O2 sterilization cycles and validated the results using biological indicators inoculated with >10 6 B. stearothermophilus. The experimental results show that in the gas phase, increasing the concentration of hydrogen peroxide and having a higher humidity level can quickly kill the microorganisms. However, the higher the concentration of hydrogen peroxide in the gas phase, the less relevant the effect of humidity level on the bactericidal effect. If the concentration of hydrogen peroxide is low, achieving the same bactericidal effect depends on higher humidity levels. Condensation invisible to the naked eye was found to shorten the sterilization time, but condensation in the visible range was not found to further enhance the bactericidal effect. The molecular deposition of water and hydrogen peroxide on the surface of the object is a determining factor for microbial deactivation, while the concentration of hydrogen peroxide in the gas phase is a secondary factor.
Key words
Condensation purification D value Bacillus stearothermophilus hydrogen peroxide inactivation isolators relative humidity steam
introduction
Hydrogen peroxide has been used as a fungicide for more than a century. In the pharmaceutical field, vaporized hydrogen peroxide has been used for the purification of barrier systems for more than a decade, but the impact of cycling parameters on the sterilization effect remains controversial and needs further investigation. This paper explores the differences in the definition of appropriate standards for relative humidity, hydrogen peroxide concentration, and temperature. There is also debate as to whether condensation is a necessary mechanism for killing microorganisms and whether the choice of dry cycle is appropriate. Currently, there are no established standard specifications or parameters for assessing the influencing factors of hydrogen peroxide sterilization. This document provides a parameter impact analysis of the vaporization hydrogen peroxide sterilization effect for open systems. The purpose of this study is to deepen understanding of the sterilization mechanism in order to optimize the sterilization cycle.
experimental design
This study conducted the following survey:
1, Tyvek (high-density polyethylene synthetic paper) sealed biological indicator D value study (four different humidity levels HLs [25% ~ 65%, 65% ~ 76%, 66% ~ 97%, 85% ~ 94% ] with three different levels of hydrogen peroxide concentration [400, 600, 800 ppm]). The factors that influence the value of D are as follows:
i. Relative humidity and hydrogen peroxide concentration
Ii. Consumption of hydrogen peroxide
Iii. Condensation
2, Tyvek (high-density polyethylene synthetic paper) sealed and unsealed biological indicator D value study (four different HLs and a hydrogen peroxide concentration level [600ppm])
3. Use the most approximate number (MPN) sterilization kinetic method (four different HLs and two different hydrogen peroxide concentration levels [400, 600 ppm])
result
A. Relative humidity and hydrogen peroxide concentration
The data reveals that high concentrations of hydrogen peroxide (equal to or above 800 ppm) and elevated humidity have no significant effect on killing. At low concentrations of hydrogen peroxide (400 ppm), higher humidity is required to achieve the same level of killing as the 800 ppm concentration.
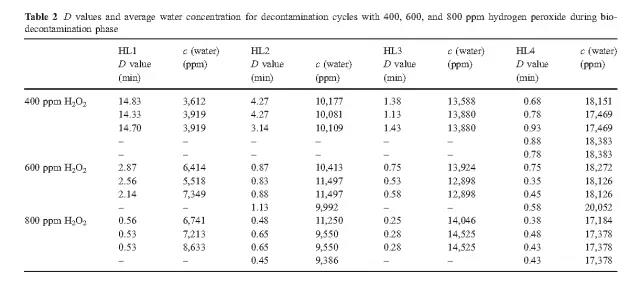
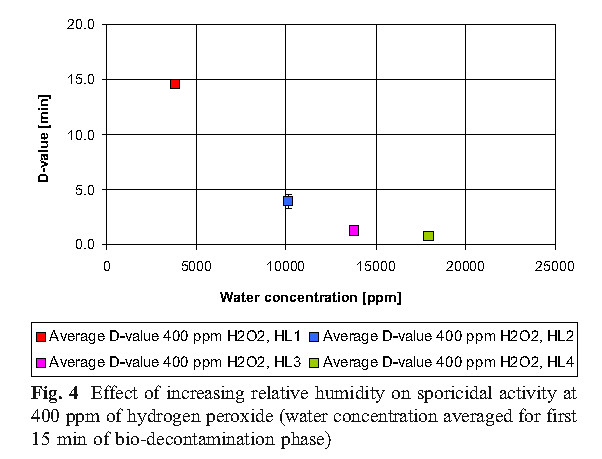
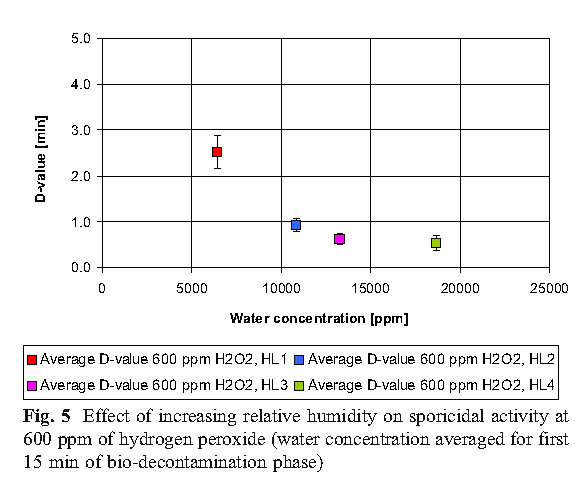
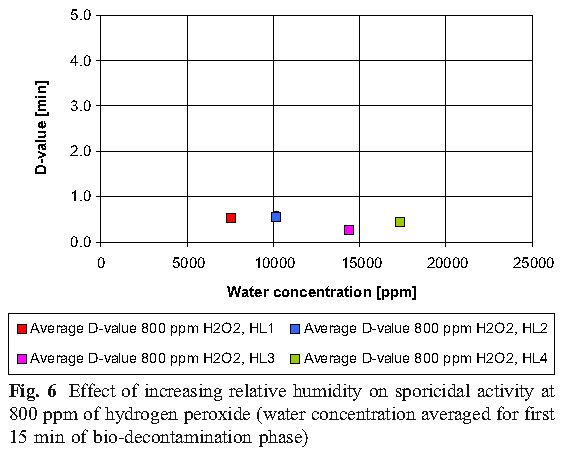
B. Hydrogen peroxide consumption
The experiment found that the most economical sterilization cycle is: 1 combination of high hydrogen peroxide concentration and low humidity (800ppmH2O2, HLs 1 and HLs 2; 2 medium hydrogen peroxide concentration and medium humidity (600ppmH2O2, HL 2); or 3 low Hydrogen peroxide concentration and high humidity (400ppmH2O2, HL3). Hydrogen peroxide vapor concentration and humidity factors together determine the bactericidal effect.

C. Condensation
The experimental results of this study show that micro-condensation is a stage of sterilization cycle when using vaporized hydrogen peroxide, and proper condensation is a requirement for effective sterilization. Therefore, in order to more effectively sterilize, it is reasonable to develop the amount of condensation required in the cycle, but there is no sufficient basis to indicate that excess condensing amount can be allowed during the cycle, because that would cause the material to be affected and the ventilation time to be prolonged.

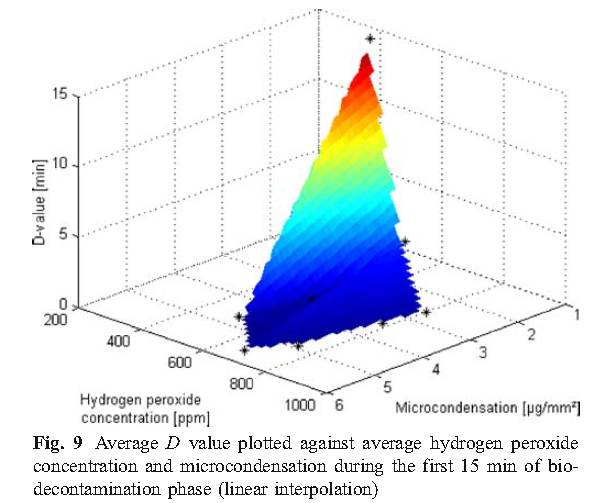

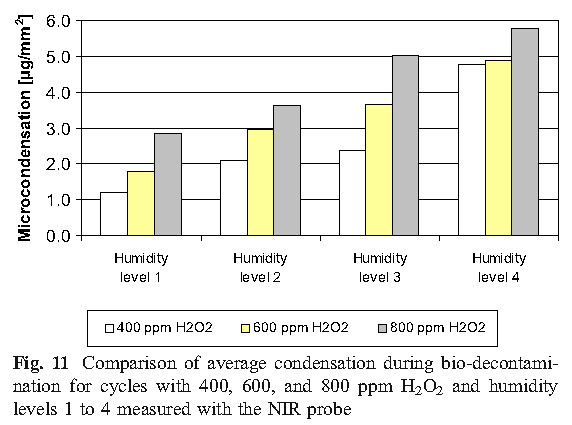
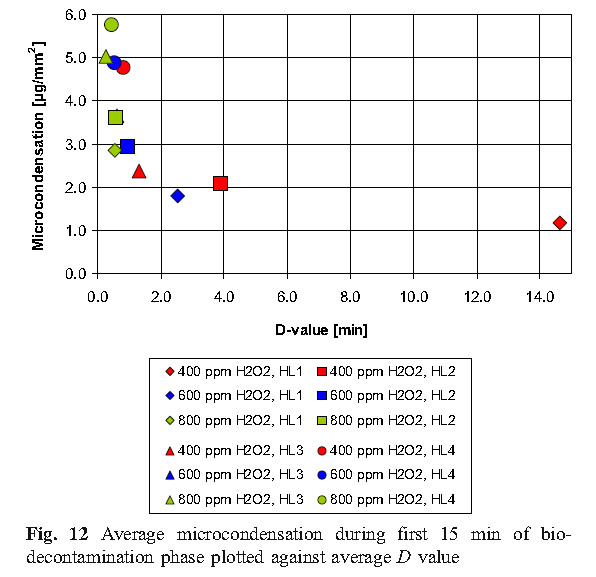
D. Values of D using Tyvek TM seal with biological indicators unsealed
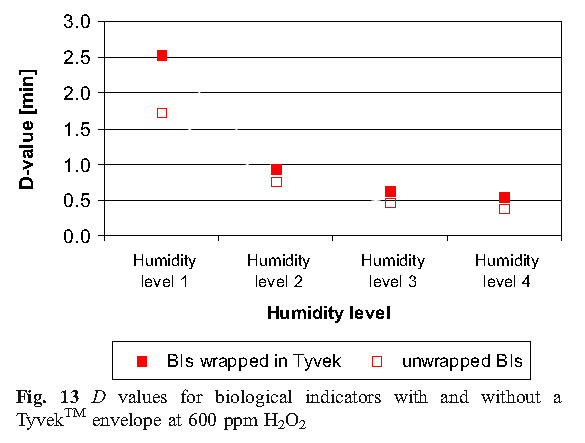
As expected, unsealed spore inactivation faster than spores using a sealed Tyvek TM, and at high humidity level conditions, all biological indicators are destroyed relatively quickly. Tyvek TM interfere with the vapor diffusion rate of biological indicators. For higher humidity levels, the D value of the unsealed and sealed biological indicator is not the same as the lower humidity level condition, and the D value is reduced from 0.8 minutes (HL1) to 0.2 minutes (HL4). As the humidity increases and the more effective condensation, the influence of the barrier Tyvek TM D value gradually decreases. Increased humidity can help promote the permeability barrier of Tyvek TM.
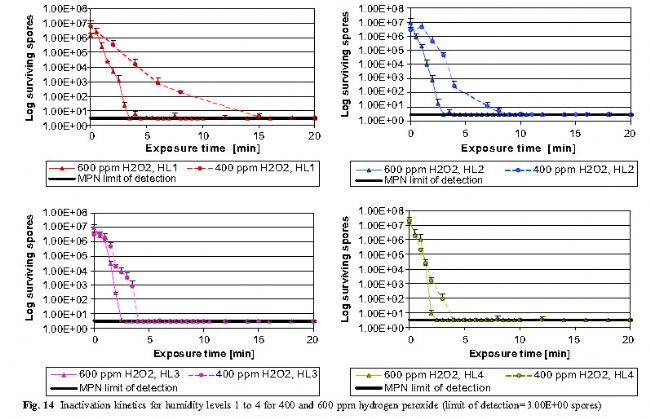
E. Spore survival curve ( MPN method)
Figure 14 shows that the sterilization rate is faster in high-concentration environments (600 ppm vs. 400 ppm H2O2), and increasing the humidity level can lead to faster inactivation of spores. When the 400 ppm H2O2 cycle is compared to the 600 ppm cycle, the different changes in the humidity range are more pronounced. 600 ppm H2O2, HL1 and 400 ppm H2O2, the HL4 cycle period demonstrates that lower hydrogen peroxide concentration conditions can achieve equivalent bactericidal effects through higher humidity levels. This result indicates that the deposition of water and hydrogen peroxide on the surface of the object is a major factor in inactivating microorganisms, and the concentration of hydrogen peroxide vapor is a secondary factor.
 to sum up
to sum up
This study shows that the successful killing of B. stearothermophilus by hydrogen peroxide vapor is a combination of several cycling parameters. Relative humidity, hydrogen peroxide vapor concentration and condensation point levels were found to be relatively sensitive during the process. High humidity and high hydrogen peroxide concentration conditions were found to increase microbial inactivation rates. Observations have shown that the primary sterilisation factor is the total deposition of water and hydrogen peroxide on the surface of the object. For a hydrogen peroxide concentration of 800 ppm, the microbial inactivation rate is not related to the humidity level. However, the lower concentration of hydrogen peroxide depends on a higher humidity environment to achieve a bactericidal effect. In contrast, higher hydrogen peroxide concentrations require only lower humidity levels. This shows that effective microbial killing can be achieved by developing different parameters. Condensation, which is visible only under the microscope, has been found to enhance the sterilization rate, but excessive condensation of water droplets needs to be avoided as it may cause material damage, prolonged ventilation, and inconsistent sterilization.
references:
[1] McDonnell G, Russell D. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev 1999;12(1):147–79.
[2] Heckert RA, et al. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl Environ Microbiol 1997;63(10):3916–8.
[3] Kokubo M, Inoue T, Akers J. Resistance of common environmental spores of the genus bacillus to vapor hydrogen peroxide. PDA J Pharm Sci Technol 1998;52(5):228–31.
SARS-CoV-2 Neutralizing Antibodies Test (ELISA)
Covid-19 Neutralizing Antibody Test,Covid-19 Neutralizing Antibodies Test,Sars-Cov-2 Neutralizing Antibody Test,Sars-Cov-2 Neutralizing Antibodies Test
Wuxi BioHermes Bio & Medical Technology Co., Ltd. , https://www.biohermesglobal.com
