Zhang Xiaoxi 1, Yue Xuan2, Li Jing1, Gu Peiming1, Wu Zeming1, Application Group, LC-MS, CMD, Japan3
1 Thermo Fisher Scientific (China) Co., Ltd.
2 Thermo Fisher Scientific, Bremen, Germany
3 Thermo Fisher Scientific, Yokohama, Japan
1 Introduction
In the last decade, Native mass spectrometry has gradually become an important technical tool for the characterization of non-covalently bound intact protein complexes [1]. For intact monoclonal antibodies (mAbs), non-denaturing mass spectrometry provides accurate measurements of molecular weight, glycoforms, etc., and can also be used for the determination of advanced structures (dimerization, trimerization, and tetramerization); non-denaturing mass spectrometry is becoming a Robust, fast and reliable first-line analytical characterization tools [2,3].
The Thermo ScientificTM ExactiveTM Plus EMR mass spectrometer, released by Thermo Fisher Scientific at the 2013 International HUPO Conference, combines unparalleled high-resolution precision quality Thermo ScientificTM OrbitrapTM analysis and extended mass range (EMR) capabilities to provide a An excellent tool for studying the structure, topology and structure of proteins and protein complexes that retain the tertiary and quaternary structure in their natural state. It can be used for the characterization of monoclonal antibody purity, PEG-modified proteins, oligomeric protein drugs, glycoforms and assembled proteins. At the same time, the comprehensive analytical performance of the Exactive Plus EMR mass spectrometer makes it the best solution for screening peptides and small molecules.
EMR's upgrade to the Exactive Plus system is reflected in:
Extended mass range m/z 350-20,000; high-quality digital ion transmission efficiency, improved signal strength; improved HCD pressure and control, easier to optimize experimental conditions; able to capture shorter time domain signals, improve Signal to noise ratio.
Figure 1 is equipped with Advion Inc. TriVersa NanoMate ®  Exactive Plus EMR for Chip Electrospray Ion Source
2 experimental methods
2.1 Sample preparation
Testing using mAb molecular weight standards (Waters 186006552), by Micro Bio-Spin® Columns (BIO- RAD) Procedure The standard system is replaced with mAb solution 100mM NH 4 Ac, mAb sample at a concentration of 10μM.
2.2 Experimental conditions
Chip-based direct injection loading conditions |
equipment* | TriVersa NanoMate® (Advion, USA) system |
Ionization voltage (kV) | 1.9 |
Air pressure (psi) | 0.3-0.6 |
*: The ESI Chip® consists of an array of 400 nanoelectrospray emitters with 5μm inner diameters. |
Mass spectrometry condition |
instrument | Exactive Plus EMR Orbitrap MS system (Figure 1) |
Mass Range | 350-20000 |
Resolution | 8750, 17500, 35000, 70000, 140000 |
Micro scans | 10 |
Insource CID energy(eV) | 100-200, optimized according to specific experimental conditions |
S-lens level (%) | 200 |
Trapping gas pressure setting factor | 3 |
Spectra average | 100 |
2.3 Data Analysis
Deconvolution analysis was performed using Protein Deconvolution 2.0 SP2.
3 results discussion
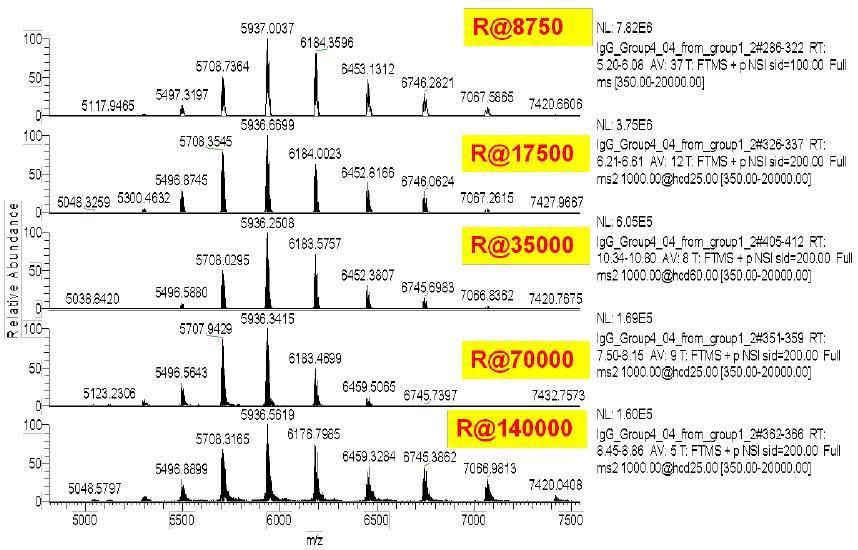
For the molecular weight test of the monoclonal antibody standard, the molecular weight under non-denaturing conditions was determined using different resolutions (8750, 17500, 35000, 70,000, 140,000) (Fig. 2).
The Exactive Plus EMR has a total of five different resolutions for different analytical purposes. It is not difficult to see from the above figure. Firstly, since the protein sample is less chargeable under non-denaturing conditions, the peak of the 150kDa monoclonal antibody is in the range of m/z 5000-8000, which is obviously higher than that under denaturing conditions. The mass end moves. The m/z limit of Exactive Plus EMR is extended to 20,000, making it easy to analyze the requirements of monoclonal antibodies and their multimers under non-denaturing conditions. Secondly, even at the lowest resolution, a high signal-to-noise ratio and a clean baseline spectrum can be acquired, which proves that the Orbitrap system has the advantages of high sensitivity, high-quality precision and high stability, which can generate high quality and high quality. The raw data of the reliability.
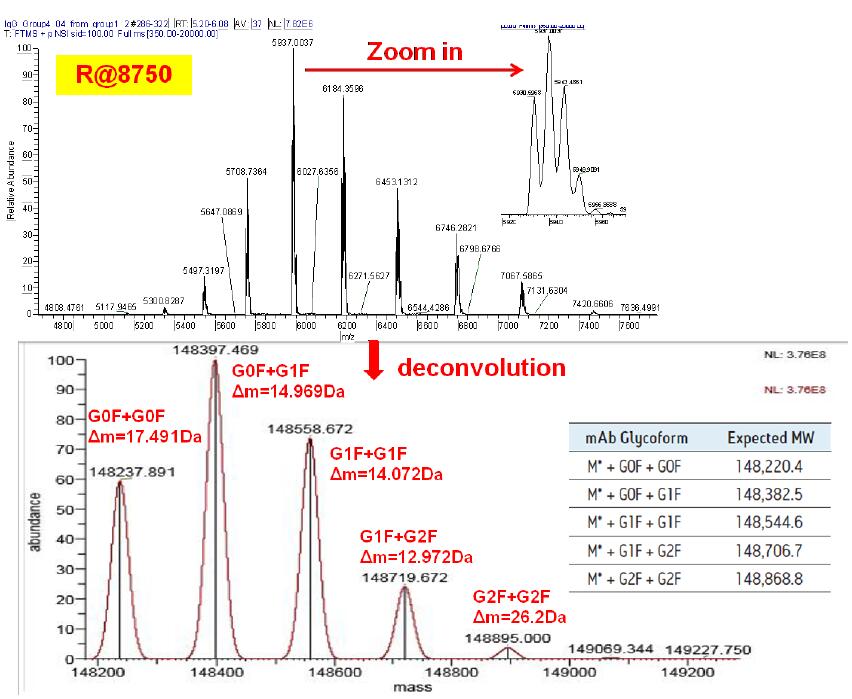
It can be seen from Fig. 3 that when the lowest resolution (8750) is used, the different glycoforms of the monoclonal antibodies cannot be separated, and the results of deconvolution can also be found, and the results are larger than the standard molecular weight. gap. This proves that when the resolution is not high enough, the deconvolution result has a large difference from the standard molecular weight due to insufficient measurement accuracy.
Figure 2 Determination of Molecular Weight of Molecular Weight Test Standards at Different Resolutions
Figure 3 Molecular weight determination results of monoclonal antibody standards at 8750 resolution
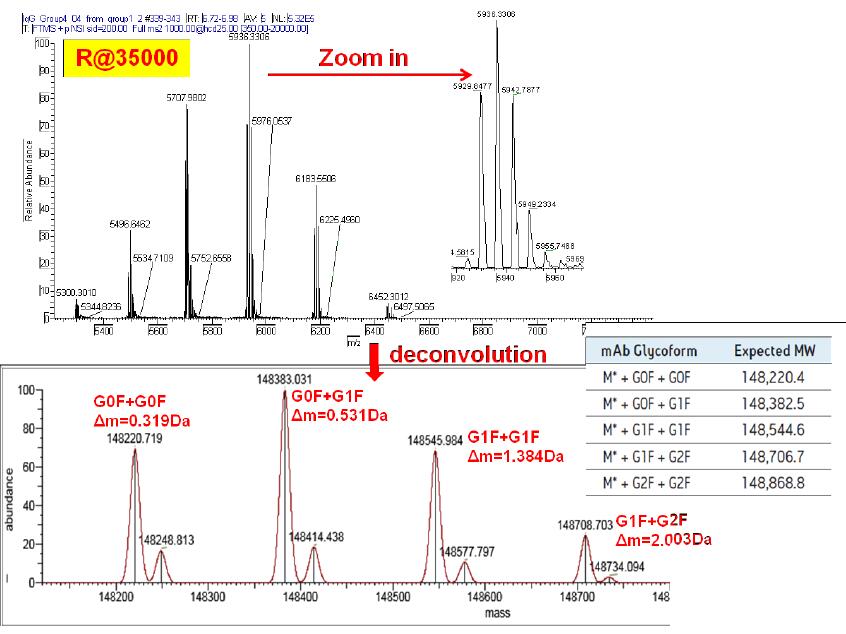
Figure 4 Molecular weight determination results of the monoclonal antibody molecular weight test standard at 35000 resolution
Figure 4 is a plot of the original and deconvoluted spectra of the molecular weight of the monoclonal antibody at 35,000 resolution. It can be seen from the original spectrum that the different glycoforms of the monoclonal antibodies were completely baseline separated, the spectra were clean, and the signal to noise ratio was high. Observing the spectrum after deconvolution, it can be found that the mass accuracy of the sample is greatly improved when the measurement accuracy is improved.
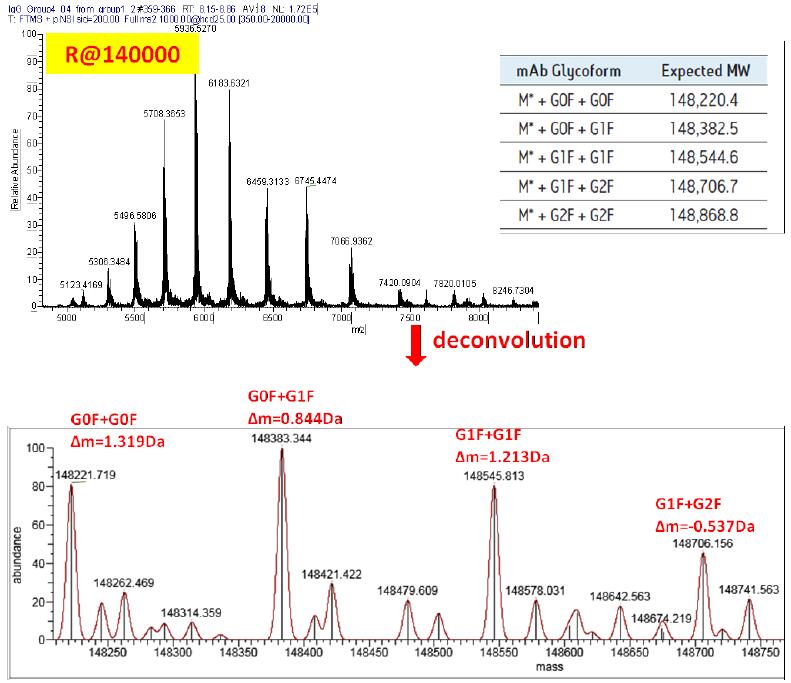
Figure 5 shows the results of molecular weight determination of monoclonal antibody standards at 140,000 resolution. As can be seen from the figure, when using the highest resolution - 140000 for analysis, many peaks that are not available at low resolution are resolved. While maintaining high resolution, Exactive Plus EMR still has extremely high sensitivity and mass accuracy. For 150kDa intact monoclonal antibodies, the main glycoforms have a mass deviation of 1.5Da (external calibration).
Figure 5 Molecular weight determination results of molecular weight test standards for single antibody at 140000 resolution
4 summary
In this experiment, we used Exactive Plus EMR high-resolution mass spectrometry to determine the molecular weight of monoclonal antibodies in non-denatured state. The results show that the Orbitrap system can achieve baseline separation between different glycoforms of monoclonal antibodies, and obtain a high-sensitivity, high-quality precision while obtaining a clear baseline separation of impurities and sample peaks, and a high signal-to-noise ratio spectrum.
It can also be observed from the experimental results that Exactive Plus EMR high-resolution mass spectrometry based on Orbitrap technology provides multiple resolutions to choose from. When the resolution is too low to separate the different modified mass spectral peaks, the resolution can be improved. They are separated to achieve high precision, high confidence results.
Non-denaturing mass spectrometry based on Exactive Plus EMR can be used not only for molecular weight determination of monoclonal antibodies, but also for drug-antibody ratio (DAR) determination, antigen-antibody binding ratio determination and protein complex analysis of ADC drugs in non-denaturing conditions. ,have a broad vision of application.
references
1 Heck, AJ Nat. Methods 2008, 5, 927-933.
2 Beck, A. et al., TrAC2013, 48, 81-95.
3 Beck, A. et al., Anal. Chem. 2013, 85, 715-36.
Monkey Pox Test Kit
Monkeypox is a viral zoonosis (a virus transmitted tothose seen in the past in smallpox patients, typically-presents clinically with fever, rash and swollen lymphnodes and may lead to a range of medical complications.It is caused by the monkeypox virus which belongs totheorthopoxvirus genus of the Poxviridae family.Thereare two clades of monkeypox virus:the West Africanclade and the Congo Basin (Central African) clade.Theoname monkeypoxoriginates from the initial discovery ofthe virus in monkeys in a Danish laboratory in 1958.Thefirst human case was identified in a child in the Demo-cratic Republic of the Congo in 1970.
Monkey Pox Test Kit,In vitro diagnostic tests,Rapid detection of monkeypox
Jiangsu iiLO Biotechnology Co., Ltd. , https://www.sjiilogene.com